PlumierideCAS# 511-89-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 511-89-7 | SDF | Download SDF |
| PubChem ID | 72319 | Appearance | Powder |
| Formula | C21H26O12 | M.Wt | 470.4 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
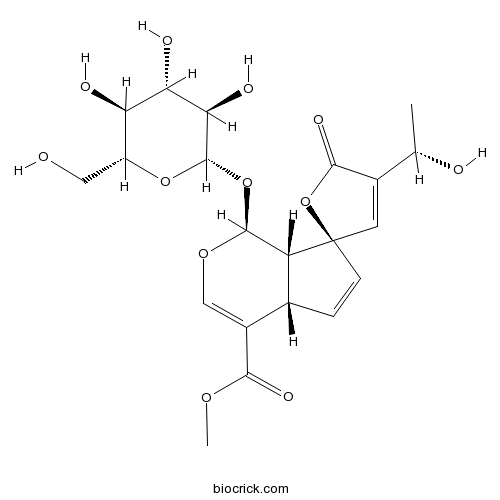
| Chemical Name | methyl (1S,4aS,7R,7aS)-4'-[(1S)-1-hydroxyethyl]-5'-oxo-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyspiro[4a,7a-dihydro-1H-cyclopenta[c]pyran-7,2'-furan]-4-carboxylate | ||
| SMILES | CC(C1=CC2(C=CC3C2C(OC=C3C(=O)OC)OC4C(C(C(C(O4)CO)O)O)O)OC1=O)O | ||
| Standard InChIKey | AOPMSFXOYJXDNJ-IRFSQMTFSA-N | ||
| Standard InChI | InChI=1S/C21H26O12/c1-8(23)10-5-21(33-18(10)28)4-3-9-11(17(27)29-2)7-30-19(13(9)21)32-20-16(26)15(25)14(24)12(6-22)31-20/h3-5,7-9,12-16,19-20,22-26H,6H2,1-2H3/t8-,9+,12+,13+,14+,15-,16+,19-,20-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Plumieride has plant growth inhibiting properties. 2. Plumieride has immunomodulatory activity. 3. Plumieride shows strong fungitoxicity against some dermatophytes causing dermatomycosis to animals and human beings. 4. Plumieride may be considered a natural antioxidant against peroxidative damage in rats. |
| Targets | Immunology & Inflammation related |

Plumieride Dilution Calculator

Plumieride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1259 mL | 10.6293 mL | 21.2585 mL | 42.517 mL | 53.1463 mL |
| 5 mM | 0.4252 mL | 2.1259 mL | 4.2517 mL | 8.5034 mL | 10.6293 mL |
| 10 mM | 0.2126 mL | 1.0629 mL | 2.1259 mL | 4.2517 mL | 5.3146 mL |
| 50 mM | 0.0425 mL | 0.2126 mL | 0.4252 mL | 0.8503 mL | 1.0629 mL |
| 100 mM | 0.0213 mL | 0.1063 mL | 0.2126 mL | 0.4252 mL | 0.5315 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vitamin D4
Catalog No.:BCC2042
CAS No.:511-28-4
- Totarol
Catalog No.:BCN4627
CAS No.:511-15-9
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
- alpha-Onocerol
Catalog No.:BCN5630
CAS No.:511-01-3
- Nicotine 1'-N-oxide
Catalog No.:BCN6892
CAS No.:51095-86-4
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- Pyrrolidinedithiocarbamate ammonium
Catalog No.:BCC6766
CAS No.:5108-96-3
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- Gitogenin
Catalog No.:BCN3886
CAS No.:511-96-6
- 8-Bromo-cGMP, sodium salt
Catalog No.:BCC6935
CAS No.:51116-01-9
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- (+)-trans-Isolimonene
Catalog No.:BCC9236
CAS No.:5113-87-1
- (S)-(+)-Ibuprofen
Catalog No.:BCC4042
CAS No.:51146-56-6
- (R)-(-)-Ibuprofen
Catalog No.:BCC4062
CAS No.:51146-57-7
- Episyringaresinol
Catalog No.:BCN7023
CAS No.:51152-20-6
- Boc-D-Asp(OBzl)-OH
Catalog No.:BCC3371
CAS No.:51186-58-4
- Withaferin A
Catalog No.:BCC7495
CAS No.:5119-48-2
- Diosgenin
Catalog No.:BCN6272
CAS No.:512-04-9
- Yamogenin
Catalog No.:BCN8277
CAS No.:512-06-1
- Raffinose
Catalog No.:BCN8427
CAS No.:512-69-6
Antioxidant Potential of Plumieride against CCl(4)-Induced Peroxidative Damage in Rats.[Pubmed:26785241]
Antioxidants (Basel). 2014 Nov 27;3(4):798-813.
In search of a new potent as an antioxidant from natural sources, Plumieride-an iridoid isolated from the methanol extract of the bark of Plumeria bicolor (family Apocynaceae) was evaluated for its antioxidant potential against CCl(4)-induced peroxidative damage in liver of rats. The antioxidant potential was evaluated by using hepatic tissue for SOD (superoxide dismutase), CAT (catalase), GSH (reduced glutathione), GPx (glutathione peroxidase), GR (glutathione reductase) and LPO (lipid peroxidation) alongwith the concomitant blood serum for AST & ALT (aspartate and alanine transaminases), GGT (gamma glutamyl transpeptidase), ALP (alkaline phosphatase), total bilirubin and total protein contents. All the biochemical parameters were significantly (p Plumieride (5, 10 and 20 mg/kg body weight/day for 30 days), restored all the parameters towards a normal level, remarkably. The histological findings of liver sections further corroborated the antioxidant potential of Plumieride compared with standard drug-silymarin. In conclusion, Plumieride consists of sugar molecules, which have alcoholic groups. Therefore, the alcoholic groups of sugar increase its antioxidant potential through intermolecular hydrogen bonding along with the thiol(SH) group of non-protein thiols and enzymes resulting in the restoration of the antioxidant system. Therefore, it might be considered a natural antioxidant against peroxidative damage in rats.
Structural modifications of plumieride isolated from Plumeria bicolor and the effect of these modifications on in vitro anticancer activity.[Pubmed:15357574]
J Org Chem. 2004 Sep 17;69(19):6165-72.
Plumieride was isolated as one of the major components from the biologically active methanolic extract of the bark of Plumeria bicolor (family Apocynaceae). For investigating the effect of substituents on cytotoxic activity it was modified into a series of compounds. Replacing the methyl ester functionality of Plumieride with alkyl amides of variable carbon units improved the cytotoxic activity, and a correlation between overall lipophilicity and cytotoxic activity was observed. In Plumieride, the glucose moiety was converted into a di- and trisaccharide by following the protection and deprotection approach, and the resulting compounds produced enhanced cytotoxicity. However, these compounds were found to be less effective than plumeiride containing a dodecyl (12 carbon units) amide group. Among all of the derivatives, the naturally occurring Plumieride showed the least cytotoxicity (50% cell kill = 49.5 microg/mL), and the dodecyl amide analogue of Plumieridepentaacetate produced the best efficacy (50% cell kill = 11.8 microg/mL). The di- and trisaccharide analogues were found to be slightly less effective than the dodecyl derivative (50% cell kill = 15-17 microg/mL). The in vitro cytotoxicity of the Plumieride analogues was determined in radiation-induced fibrosarcoma (RIF) tumor cells.
Plumieride from Allamanda cathartica as an antidermatophytic agent.[Pubmed:12112301]
Phytother Res. 2002 Jun;16(4):393-4.
Plumieride has been isolated as an active principle of the leaves of Allamanda cathartica. It showed strong fungitoxicity against some dermatophytes causing dermatomycosis to animals and human beings. It exhibited a noncytotoxic nature against a P(388) mouse leukaemia cell line.
Effects of plumieride, an iridoid on spermatogenesis in male albino rats.[Pubmed:15070168]
Phytomedicine. 2004 Feb;11(2-3):169-74.
UNLABELLED: Oral feeding of male rats with Plumieride (15 mg/rat/day) for the period of 60 days did not cause any significant change in the body weight of treated rats. However, the weights of testes, epididymides, seminal vesicle and ventral prostate were significantly reduced when compared to control values. The production of step-19 spermatids was reduced by 87.26% in Plumieride treated rats. The population of preleptotene and pachytene spermatocytes were decreased by 64.26% and 55.13% respectively. Spermatogonia and sertoli cell population was also affected. Plumieride treatment resulted in an arrest of spermatogenesis without any systemic side effect. Sperm motility as well as sperm density was reduced significantly. The number of mature Leydig cells was decreased and complete suppression of fertility was observed. A significant fall in the protein and sialic acid contents of the testes, epididymides, seminal vesicle and ventral prostate as well as glycogen content of testes was also noticed. Fructose in seminal vesicle was lowered whereas testicular cholesterol was elevated. There was no significant change in RBC and WBC count, haemoglobin, haematocrit and sugar in the whole blood and total protein, cholesterol, phospholipid and triglycerides in the serum. CONCLUSION: Plumieride administration arrests spermatogenesis in male rats without noticeable side effects. For the clinical use more experiments should be carried out in a phased programme.


