Physalin BCAS# 23133-56-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23133-56-4 | SDF | Download SDF |
| PubChem ID | 11730919 | Appearance | Powder |
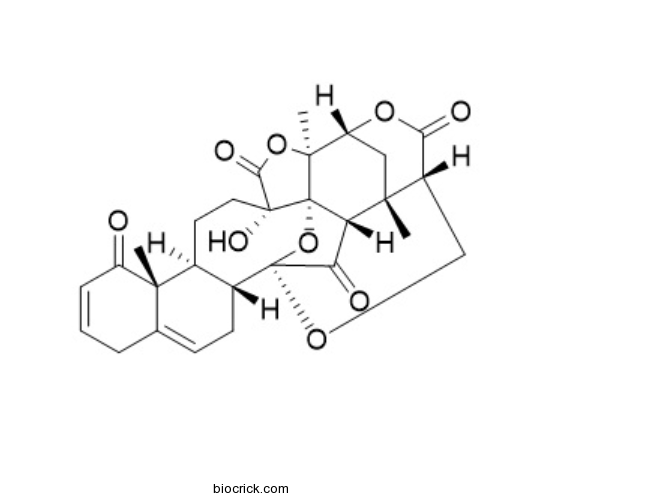
| Formula | C28H30O9 | M.Wt | 510.53 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2S,5S,8S,9R,17R,18R,21S,24R,26S,27S)-5-hydroxy-2,9,26-trimethyl-3,19,23,28-tetraoxaoctacyclo[16.9.1.118,27.01,5.02,24.08,17.09,14.021,26]nonacosa-11,14-diene-4,10,22,29-tetrone | ||
| SMILES | CC12CC3C4(C56C1C(=O)C(O5)(C7CC=C8CC=CC(=O)C8(C7CCC6(C(=O)O4)O)C)OCC2C(=O)O3)C | ||
| Standard InChIKey | HVTFEHJSUSPQBK-IIOAQAAUSA-N | ||
| Standard InChI | InChI=1S/C28H30O9/c1-23-11-18-25(3)28-19(23)20(30)27(37-28,34-12-16(23)21(31)35-18)15-8-7-13-5-4-6-17(29)24(13,2)14(15)9-10-26(28,33)22(32)36-25/h4,6-7,14-16,18-19,33H,5,8-12H2,1-3H3/t14-,15+,16-,18+,19-,23+,24-,25-,26+,27+,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Physalin B shows potent anti-Trypanosoma cruzi activity. 2. Physalin B shows antimalarial activity. 3. Physalin B can inhibit the growth of several human leukemia cells. 4. Physalin B has the potential to be developed as an effective chemotherapeutic lead compound for the treatment of malignant melanoma. 5. Physalin B inhibits Rhodnius prolixus hemocyte phagocytosis and microaggregation by the activation of endogenous PAF-acetyl hydrolase activities. 6. Physalin B exhibits a minimum inhibitory concentration value (MIC) against Mycobacterium tuberculosis H(37)Rv strain of 32 microg/mL. 7. Physalin B inhibits androgen-independent prostate cancer cell growth through activation of cell apoptosis and downregulation of androgen receptor expression. 8. Physalin B presents antinociceptive properties associated with central. |
| Targets | Bcl-2/Bax | Caspase | Antifection | PAFR | JNK | ERK | TNF-α |

Physalin B Dilution Calculator

Physalin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9587 mL | 9.7937 mL | 19.5875 mL | 39.175 mL | 48.9687 mL |
| 5 mM | 0.3917 mL | 1.9587 mL | 3.9175 mL | 7.835 mL | 9.7937 mL |
| 10 mM | 0.1959 mL | 0.9794 mL | 1.9587 mL | 3.9175 mL | 4.8969 mL |
| 50 mM | 0.0392 mL | 0.1959 mL | 0.3917 mL | 0.7835 mL | 0.9794 mL |
| 100 mM | 0.0196 mL | 0.0979 mL | 0.1959 mL | 0.3917 mL | 0.4897 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Heveaflavone
Catalog No.:BCN5079
CAS No.:23132-13-0
- Tectorigenin 7-O-xylosylglucoside
Catalog No.:BCN2903
CAS No.:231288-19-0
- 6'-O-xylosyl-glycitin
Catalog No.:BCN8169
CAS No.:231288-18-9
- Mudanpioside H
Catalog No.:BCC9049
CAS No.:231280-71-0
- 5-(4-((3-chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl)furan-2-carbaldehyde
Catalog No.:BCC8719
CAS No.:231278-84-5
- N-[3-Chloro-4-(3-fluorobenzyloxy)phenyl]-6-iodoquinazolin-4-amine
Catalog No.:BCC9068
CAS No.:231278-20-9
- Lapatinib
Catalog No.:BCC3633
CAS No.:231277-92-2
- Methylxanthoxylin
Catalog No.:BCC8212
CAS No.:23121-32-6
- Fumagillin
Catalog No.:BCC2347
CAS No.:23110-15-8
- UK 370106
Catalog No.:BCC2379
CAS No.:230961-21-4
- UK 356618
Catalog No.:BCC2378
CAS No.:230961-08-7
- Neuropeptide SF (mouse, rat)
Catalog No.:BCC6054
CAS No.:230960-31-3
- Strictosamide
Catalog No.:BCN5080
CAS No.:23141-25-5
- Vincosamide
Catalog No.:BCN5081
CAS No.:23141-27-7
- Pentoxyverine Citrate
Catalog No.:BCC4697
CAS No.:23142-01-0
- Oxymetazoline HCl
Catalog No.:BCC4333
CAS No.:2315-02-8
- H-Glu(OMe)-OMe.HCl
Catalog No.:BCC2932
CAS No.:23150-65-4
- 3,4-Dimethoxycinnamic acid
Catalog No.:BCN5040
CAS No.:2316-26-9
- Pyrolatin
Catalog No.:BCN8439
CAS No.:23176-70-7
- Nervosine
Catalog No.:BCN2012
CAS No.:23179-26-2
- Songoramine
Catalog No.:BCN6474
CAS No.:23179-78-4
- Senkirkine
Catalog No.:BCN2136
CAS No.:2318-18-5
- Simiarenone
Catalog No.:BCN5082
CAS No.:2318-78-7
- Paeoniflorin
Catalog No.:BCN6301
CAS No.:23180-57-6
Antinociceptive properties of physalins from Physalis angulata.[Pubmed:25396337]
J Nat Prod. 2014 Nov 26;77(11):2397-403.
Pain is the most common reason a patient sees a physician. Nevertheless, the use of typical painkillers is not completely effective in controlling all pain syndromes; therefore further attempts have been made to develop improved analgesic drugs. The present study was undertaken to evaluate the antinociceptive properties of physalins B (1), D (2), F (3), and G (4) isolated from Physalis angulata in inflammatory and centrally mediated pain tests in mice. Systemic pretreatment with 1-4 produced dose-related antinociceptive effects on the writhing and formalin tests, traditional screening tools for the assessment of analgesic drugs. On the other hand, only 3 inhibited inflammatory parameters such as hyperalgesia, edema, and local production of TNF-alpha following induction with complete Freund's adjuvant. Treatment with 1, 3, and 4 produced an antinociceptive effect on the tail flick test, suggesting a centrally mediated antinociception. Reinforcing this idea, 2-4 enhanced the mice latency reaction time during the hot plate test. Mice treated with physalins did not demonstrate motor performance alterations. These results suggest that 1-4 present antinociceptive properties associated with central, but not anti-inflammatory, events and indicate a new pharmacological property of physalins.
Antimalarial activity of physalins B, D, F, and G.[Pubmed:21954931]
J Nat Prod. 2011 Oct 28;74(10):2269-72.
The antimalarial activities of physalins B, D, F, and G (1-4), isolated from Physalis angulata, were investigated. In silico analysis using the similarity ensemble approach (SEA) database predicted the antimalarial activity of each of these compounds, which were shown using an in vitro assay against Plasmodium falciparum. However, treatment of P. berghei-infected mice with 3 increased parasitemia levels and mortality, whereas treatment with 2 was protective, causing a parasitemia reduction and a delay in mortality in P. berghei-infected mice. The exacerbation of in vivo infection by treatment with 3 is probably due to its potent immunosuppressive activity, which is not evident for 2.
Physalin B from Physalis angulata triggers the NOXA-related apoptosis pathway of human melanoma A375 cells.[Pubmed:22245079]
Food Chem Toxicol. 2012 Mar;50(3-4):619-24.
Melanoma is a lethal form of skin cancer that can metastasize rapidly. While surgery and radiation therapy provide palliative therapy for local tumor growth, systemic therapy is the mainstay of treatment for metastatic melanoma. However, limited chemotherapeutic agents are available for melanoma treatment. In this study, we investigated the anti-melanoma effect of Physalin B, the major active compound from a widely used herb medicine, Physalis angulata L. This study demonstrated that Physalin B exhibits cytotoxicity towards v-raf murine sarcoma viral oncogene homolog B1 (BRAF)-mutated melanoma A375 and A2058 cells (the IC50 values are lower than 4.6 mug/ml). Cytotoxicity is likely resulted from apoptosis since the apoptotic marker phosphatidylserine are detected immediately under Physalin B treatment and apoptotic cells formation. Further examination revealed that Physalin B induces expression of the proapoptotic protein NOXA within 2 h and later triggers the expression of Bax and caspase-3 in A375 cells. These results indicate that Physalin B can induce apoptosis of melanoma cancer cells via the NOXA, caspase-3, and mitochondria-mediated pathways, but not of human skin fibroblast cells and myoblastic cells. Thus, Physalin B has the potential to be developed as an effective chemotherapeutic lead compound for the treatment of malignant melanoma.
Physalins A and B inhibit androgen-independent prostate cancer cell growth through activation of cell apoptosis and downregulation of androgen receptor expression.[Pubmed:21963499]
Biol Pharm Bull. 2011;34(10):1584-8.
Androgen deprivation therapy is a common treatment strategy for advanced prostate cancer. Though effective initially, the tumor often progresses to androgen independent stage in most patients eventually after a period of remission. One of the key factors of development of resistance is reflected in expression of androgen receptor (AR). In this study, we showed that two natural compounds, physalins A and B, both secosteriods from Physalisalkekengi var. franchetii, significantly inhibited the growth of two androgen-independent cell lines CWR22Rv1 and C42B, induced apoptosis via c-Jun N-terminal kinase (JNK) and/or extracellular signal-regulated kinase (ERK) activation, and decreased AR expression. In addition, physalins A and B down-regulated the expression of prostate specific antigen (PSA) in C42B cells which is a target gene of AR. Our results suggest that physalin A and B might be useful agents in preventing the growth of androgen-independent prostate cancer (AI-PCa).
Inhibitory effects of physalin B and physalin F on various human leukemia cells in vitro.[Pubmed:1503404]
Anticancer Res. 1992 Jul-Aug;12(4):1155-62.
Physalins B and F were isolated and characterized from the ethanolic extract of the whole plant of Physalis angulata L. (Solanaceae). Both Physalin B and physalin F inhibited the growth of several human leukemia cells: K562 (erythroleukemia), APM1840 (acute T lymphoid leukemia), HL-60 (acute promyelocytic leukemia), KG-1 (acute myeloid leukemia), CTV1 (acute monocytic leukemia) and B cell (acute B lymphoid leukemia). Physalin F showed a stronger activity against these leukemia cells than Physalin B, especially against acute myeloid leukemia (KG-1) and acute B lymphoid leukemia (B cell). From the structural features, the active site seems to be the functional epoxy group for physalin F and the double bond for Physalin B located at carbon 5 and 6; the former is much more active than the latter as regards anti-leukemic effects.
Physalins B and F, seco-steroids isolated from Physalis angulata L., strongly inhibit proliferation, ultrastructure and infectivity of Trypanosoma cruzi.[Pubmed:24001147]
Parasitology. 2013 Dec;140(14):1811-21.
We previously observed that physalins have immunomodulatory properties, as well as antileishmanial and antiplasmodial activities. Here, we investigated the anti-Trypanosoma cruzi activity of physalins B, D, F and G. We found that physalins B and F were the most potent compounds against trypomastigote and epimastigote forms of T. cruzi. Electron microscopy of trypomastigotes incubated with Physalin B showed disruption of kinetoplast, alterations in Golgi apparatus and endoplasmic reticulum, followed by the formation of myelin-like figures, which were stained with MDC to confirm their autophagic vacuole identity. Physalin B-mediated alteration in Golgi apparatus was likely due to T. cruzi protease perturbation; however physalins did not inhibit activity of the trypanosomal protease cruzain. Flow cytometry examination showed that cell death is mainly caused by necrosis. Treatment with physalins reduced the invasion process, as well as intracellular parasite development in macrophage cell culture, with a potency similar to benznidazole. We observed that a combination of physalins and benznidazole has a greater anti-T. cruzi activity than when compounds were used alone. These results indicate that physalins, specifically B and F, are potent and selective trypanocidal agents. They cause structural alterations and induce autophagy, which ultimately lead to parasite cell death by a necrotic process.
Physalin B inhibits Rhodnius prolixus hemocyte phagocytosis and microaggregation by the activation of endogenous PAF-acetyl hydrolase activities.[Pubmed:19232405]
J Insect Physiol. 2009 Jun;55(6):532-7.
The effects of Physalin B (a natural secosteroidal chemical from Physalis angulata, Solanaceae) on phagocytosis and microaggregation by hemocytes of 5th-instar larvae of Rhodnius prolixus were investigated. In this insect, hemocyte phagocytosis and microaggregation are known to be induced by the platelet-activating factor (PAF) or arachidonic acid (AA) and regulated by phospholipase A(2) (PLA(2)) and PAF-acetyl hydrolase (PAF-AH) activities. Phagocytic activity and formation of hemocyte microaggregates by Rhodnius hemocytes were strongly blocked by oral treatment of this insect with Physalin B (1mug/mL of blood meal). The inhibition induced by Physalin B was reversed for both phagocytosis and microaggregation by exogenous arachidonic acid (10microg/insect) or PAF (1microg/insect) applied by hemocelic injection. Following treatment with Physalin B there were no significant alterations in PLA(2) activities, but a significant enhancement of PAF-AH was observed. These results show that Physalin B inhibits hemocytic activity by depressing insect PAF analogous (iPAF) levels in hemolymph and confirm the role of PAF-AH in the cellular immune reactions in R. prolixus.
Antimycobacterial physalins from Physalis angulata L. (Solanaceae).[Pubmed:12203265]
Phytother Res. 2002 Aug;16(5):445-8.
Crude extracts and fractions from aerial parts of Physalis angulata have been bioassayed for antimycobacterial activity. Fraction A1-29-12 containing physalins B, F and D exhibited a minimum inhibitory concentration value (MIC) against Mycobacterium tuberculosis H(37)Rv strain of 32 microg/mL. Purified Physalin B and physalin D were also tested showing MIC values against Mycobacterium tuberculosis H(37)Rv strain of > 128 microg/mL and 32 microg/mL respectively, suggesting that physalin D plays a relevant role in the antimycobacterial activity displayed. Structural elucidation of both physalins D and B was based on detailed (13)C and (1)H NMR spectral analysis with the aid of 2D-correlation spectroscopy ((1)H-(1)H, COSY, HSQC and HMBC). The assignment of the (13)C chemical shift for physalin D is reported here for the first time.


