PhortressProdrug of antitumor agent 5F 203 CAS# 328087-38-3 |
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

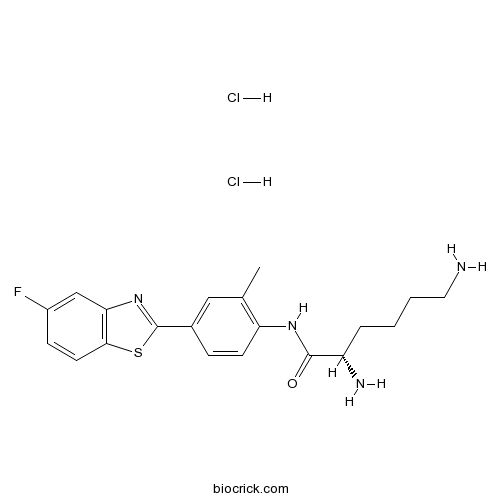
| Cas No. | 328087-38-3 | SDF | Download SDF |
| PubChem ID | 9804228 | Appearance | Powder |
| Formula | C20H25Cl2FN4OS | M.Wt | 459.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | (2S)-2,6-diamino-N-[4-(5-fluoro-1,3-benzothiazol-2-yl)-2-methylphenyl]hexanamide;dihydrochloride | ||
| SMILES | CC1=C(C=CC(=C1)C2=NC3=C(S2)C=CC(=C3)F)NC(=O)C(CCCCN)N.Cl.Cl | ||
| Standard InChIKey | QZSMNTOCJVVFEU-CKUXDGONSA-N | ||
| Standard InChI | InChI=1S/C20H23FN4OS.2ClH/c1-12-10-13(20-25-17-11-14(21)6-8-18(17)27-20)5-7-16(12)24-19(26)15(23)4-2-3-9-22;;/h5-8,10-11,15H,2-4,9,22-23H2,1H3,(H,24,26);2*1H/t15-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Prodrug of the antitumor agent 5F 203, which acts via binding to aryl hydrocarbon receptors. Induces expression of CYP1A1 and generates adducts in the DNA of sensitive MCF7 and IGROV-1 cells. |

Phortress Dilution Calculator

Phortress Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1767 mL | 10.8835 mL | 21.767 mL | 43.5341 mL | 54.4176 mL |
| 5 mM | 0.4353 mL | 2.1767 mL | 4.3534 mL | 8.7068 mL | 10.8835 mL |
| 10 mM | 0.2177 mL | 1.0884 mL | 2.1767 mL | 4.3534 mL | 5.4418 mL |
| 50 mM | 0.0435 mL | 0.2177 mL | 0.4353 mL | 0.8707 mL | 1.0884 mL |
| 100 mM | 0.0218 mL | 0.1088 mL | 0.2177 mL | 0.4353 mL | 0.5442 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that promotes the expression of phase I and II xenobiotic chemical metabolizing enzyme genes, including the cytochrome P450 (CYP) isoforms CYP1A1 and CYP1A2. Phortress is a lysyl amide prodrug of the benzothiazole 5-fluoro 203 , a high affinity AhR ligand that elicits antitumor activity by inducing transcription of CYP1A1, which leads to the formation of DNA adducts and cell cycle arrest. Phortress rapidly reverts to 5-fluoro 203 in carcinoma cell lines, resulting in significant growth inhibition at nanomolar concentrations. At 20 mg/kg, phortress can suppress the growth of breast and ovarian xenografts in vivo.
- Ceranib 1
Catalog No.:BCC6186
CAS No.:328076-61-5
- H-D-Leu-OH
Catalog No.:BCC2975
CAS No.:328-38-1
- Nomifensine
Catalog No.:BCC7226
CAS No.:32795-47-4
- Panaxatriol
Catalog No.:BCN1081
CAS No.:32791-84-7
- Labetalol HCl
Catalog No.:BCC5489
CAS No.:32780-64-6
- Protopanaxatriol
Catalog No.:BCC9245
CAS No.:32773-56-1
- Macrocarpal O
Catalog No.:BCN7371
CAS No.:327622-65-1
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
- Macrocarpal L
Catalog No.:BCN5248
CAS No.:327601-97-8
- Phorbol 13-acetate
Catalog No.:BCN7231
CAS No.:32752-29-7
- Heliosupine
Catalog No.:BCN1980
CAS No.:32728-78-2
- tcY-NH2
Catalog No.:BCC5770
CAS No.:327177-34-4
- Coniferyl alcohol
Catalog No.:BCN4651
CAS No.:32811-40-8
- (H-Cys-OMe)2.2HCl
Catalog No.:BCC2916
CAS No.:32854-09-4
- Lannaconitine
Catalog No.:BCN2504
CAS No.:32854-75-4
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- AG-14361
Catalog No.:BCC2209
CAS No.:328543-09-5
- Benzylamine hydrochloride
Catalog No.:BCN6908
CAS No.:3287-99-8
- Cajanin
Catalog No.:BCN5249
CAS No.:32884-36-9
- Lasiodiplodin
Catalog No.:BCN4770
CAS No.:32885-81-7
- De-O-methyllasiodiplodin
Catalog No.:BCN7187
CAS No.:32885-82-8
- Tildipirosin
Catalog No.:BCC5478
CAS No.:328898-40-4
- pep2-SVKI
Catalog No.:BCC5784
CAS No.:328944-75-8
- C646
Catalog No.:BCC4546
CAS No.:328968-36-1
The experimental antitumor agents Phortress and doxorubicin are equiactive against human-derived breast carcinoma xenograft models.[Pubmed:15377855]
Breast Cancer Res Treat. 2004 Sep;87(1):97-107.
Phortress (the dihydrochloride salt of the lysylamide prodrug of 2-(4-amino-3-methylphenyl)-5-fluoro-benzothiazole (5F 203)) is an experimental antitumor agent with potent and selective activity against human-derived carcinomas of breast, ovarian and renal origin. UK clinical trials of Phortress are scheduled to begin in 2004. The mechanism of action of Phortress is distinct from all classes of chemotherapeutic agents currently in the clinic, and involves metabolic activation by cytochrome P450 (CYP) 1A1 to electrophilic species, which generate DNA adducts in sensitive tumors only. In the present study, the antitumor efficacy of Phortress has been compared with that of doxorubicin (Dox) in nine human-derived mammary carcinoma xenograft models, cultivated subcutaneously in the flanks of nude mice. In addition, cyp1a1 mRNA expression was measured in tumors of control and treated animals. Phortress compared favorably with Dox: significant activity, independent of estrogen receptor (ER) status, was established in 7/9 xenografts; in one xenograft model, Phortress elicited superior antitumor activity; no model demonstrated complete resistance to Phortress. In accordance with this observation, all xenografts available for examination (8) displayed clear induction of cyp1a1 expression upon treatment of mice with Phortress whereas Dox failed to induce cyp1a1 expression in all models. Prolonged viability of tumor fragments, recovered for treatment ex vivo could not be sustained; thus correlations between tumor cells' response to Phortress and cyp1a1 or cyp1b1 inducibility following 5F 203 treatment could not be determined with confidence.
Preclinical toxicokinetic evaluation of phortress [2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride] in two rodent species.[Pubmed:19088497]
Pharmacology. 2009;83(2):99-109.
BACKGROUND AND AIMS: The 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole prodrug Phortress exerts potent and selective antitumour activity in vitro and in vivo. Preclinical toxicokinetic studies in 2 rodent species were undertaken to determine Phortress' maximum tolerated dose and advise a safe starting dose for clinical evaluation. METHODS: Plasma pharmacokinetic parameters were determined by high-performance liquid chromatography and fluorescence detection following Phortress administration to mice (10 mg/kg, intravenously on days 1 and 8). Phortress (20 mg/kg, on days 1 and 8) was administered to CYP1A1/betaGAL reporter mice; tissues were examined macro- and microscopically. Toxicological and pharmacodynamic endpoints were examined in organs of rodents receiving Phortress (10 mg/kg or 20 mg/kg, on days 1 and 8). CYP1A1 expression and Phortress-derived DNA adducts were determined in lungs and livers (on days 11 and 36). RESULTS: No accumulation of Phortress was detected in murine plasma. beta-Galactosidase activity inferred Phortress-derived induction of cyp1a1 transcription in the livers of transgenic mice; no total body weight loss was encountered in these animals. However, a fall in lung:body weight and kidney:body weight ratios, raised serum alkaline phosphatase levels and hepatic histopathological disturbances in animals receiving 20 mg/kg Phortress indicate organ sites of potential toxicity. CYP1A1 protein was induced transiently in the lungs of both species and in the livers of rats. Elimination of hepatic DNA adducts and rat pulmonary adducts was evident; however, murine pulmonary adducts persisted. CONCLUSION: Rodent preclinical toxicology established that mice represent the more sensitive rodent species, resolving a maximum tolerated dose of 10 mg/kg Phortress.
In vitro exposure of precision-cut lung slices to 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride (NSC 710305, Phortress) increases inflammatory cytokine content and tissue damage.[Pubmed:23143926]
Toxicol Sci. 2013 Feb;131(2):470-9.
The anticancer drug (2-[4-amino-3-methylphenyl]-5-fluorobenzothiazole lysylamide dihydrochloride) (NSC 710305, Phortress) is a metabolically activated prodrug that causes DNA adduct formation and subsequent toxicity. Preclinically, it was found that hepatic, bone marrow, and pulmonary toxicity presented challenges to developing this drug. An ex vivo precision-cut lung slice (PCLS) model was used to search for concentration dependent effects of NSC 710305 (10, 25, 50, and 100 microM) on cytokine content, protein content, and immuno/histological endpoints. Preparation and culture of PCLS caused an initial spike in proinflammatory cytokine expression and therefore treatment with NSC 710305 was delayed until 48 h after initiating the slice cultures to avoid confounding the response to slicing with any drug response. PCLSs were evaluated after 24, 48, and 72 h exposures to NSC 710305. Reversibility of toxicity due to the 72-h treatment was evaluated after a 24-h recovery period. NSC 710305 caused a concentration-dependent cytokine response, and only the toxicity caused by a 72-h exposure to 25 microM reversed during the 24-h recovery period. Immuno/histological examination and quantitation of tissue protein levels indicated that tissue destruction, ED-1 (activated macrophage) staining, and protein levels were associated with the levels of proinflammatory cytokines in the tissue. In conclusion, the concentration- and time-dependent inflammatory response of PCLS to NSC 710305 preceded relevant tissue damage by a few days. The no-observable adverse effect level (NOAEL) for 24, 48, and 72 h exposures was established as 10 microM NSC 710305.
In vitro cytotoxicity of Phortress against colorectal cancer.[Pubmed:17016663]
Int J Oncol. 2006 Nov;29(5):1287-94.
Phortress is a novel benzothiazole compound with activity concentrated in certain breast, ovarian and renal cancer cell lines. Its anti-angiogenic effects are unknown. In this study, the in vitro anti-angiogenic effects of Phortress were screened for and results compared with two control drugs, paclitaxel and fumagillin. in vitro anti-angiogenic activity was examined by MTS assays, growth curves and clonogenic survival assays on human umbilical vein endothelial cells (HUVEC). In addition and as a comparator, effects were examined on MRCV fibroblasts and also the MCF7 breast cancer cell line, shown to be sensitive on the NCI60 panel and 3 colorectal cancer cell lines (HT29, SW480 and SW620) that were reportedly insensitive. Effects on endothelial tube differentiation were assessed by the Matrigel assay. Phortress had no effect on HUVEC and MRCV cell proliferation and survival. Unlike paclitaxel and fumagillin, Phortress did not inhibit endothelial tube differentiation. Phortress therefore exhibits no in vitro anti-angiogenic activity. As expected, Phortress was cytotoxic to MCF7 breast cancer cells, but unexpectedly, Phortress was also potent against colorectal cancer cells in clonogenic survival and cell growth (growth curves but not MTS assay) end-points. The efficacy of Phortress against colorectal cancer cells in the current study confirms that the spectrum of activity of Phortress may be wider than previously thought.
The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate.[Pubmed:15078163]
Curr Med Chem. 2004 Apr;11(8):1009-21.
This review traces the development of a series of potent and selective antitumour benzothiazoles from the discovery of the initial lead compound, 2-(4-amino-3-methylphenyl)benzothiazole (DF 203) in 1995 to the identification of a clinical candidate, Phortress, scheduled to enter Phase 1 trials in Q1 2004 under the auspices of Cancer Research U.K. Advances in our understanding of the mechanism of action of this unique series of agents are described and can be summarised as follows: selective uptake into sensitive cells followed by Arylhydrocarbon Receptor (AhR) binding and translocation into the nucleus, induction of the cytochrome P450 isoform (CYP) 1A1, conversion of the drug into an electrophilic reactive intermediate and formation of extensive DNA adducts resulting in cell death. Our understanding of this mechanistic scenario has played a crucial role in the drug development process, most notably in the synthesis of fluorinated DF 203 analogues to thwart deactivating oxidative metabolism (5F 203) and water-soluble prodrug design for parenteral administration. Aspects of mechanism of action studies, in vitro and in vivo screening, synthetic chemistry and pharmacokinetics are reviewed here.
In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles.[Pubmed:15634650]
Mol Cancer Ther. 2004 Dec;3(12):1565-75.
Phortress is a novel, potent, and selective experimental antitumor agent. Its mechanism of action involves induction of CYP1A1-catalyzed biotransformation of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203) to generate electrophilic species, which covalently bind to DNA, exacting lethal damage to sensitive tumor cells, in vitro and in vivo. Herein, we investigate the effects of DNA adduct formation on cellular DNA integrity and progression through cell cycle and examine whether a relevant pharmacodynamic end point may be exploited to probe the clinical mechanism of action of Phortress and predict tumor response. Single cell gel electrophoresis (SCGE) was applied to quantify DNA damage and cell cycle analyses conducted upon 5F 203 treatment of benzothiazole-sensitive MCF-7 and inherently resistant MDA-MB-435 breast carcinoma cells. Following treatment of xenograft-bearing mice and mice possessing hollow fiber implants containing MCF-7 or MDA-MB-435 cells with Phortress (20 mg/kg, i.p., 24 hours), tumor cells and xenografts were recovered for analyses by SCGE. Dose- and time-dependent DNA single and double strand breaks occurred exclusively in sensitive cells following treatment with 5F 203 in vitro (10 nmol/L-10 micromol/L; 24-72 hours). In vivo, Phortress-sensitive and Phortress-resistant tumor cells were distinct; moreover, DNA damage in xenografts, following treatment of mice with Phortress, could be determined. Interrogation of the mechanism of action of 5F 203 in silico by self-organizing map-based cluster analyses revealed modulation of phosphatases and kinases associated with cell cycle regulation, corroborating observations of selective cell cycle perturbation by 5F 203 in sensitive cells. By conducting SCGE, tumor sensitivity to Phortress, an agent currently undergoing clinical evaluation, may be determined.
Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo.[Pubmed:12569393]
Br J Cancer. 2003 Feb 10;88(3):470-7.
2-(4-Aminophenyl)benzothiazoles represent a potent and highly selective class of antitumour agent. In vitro, sensitive carcinoma cells deplete 2-(4-aminophenyl)benzothiazoles from nutrient media; cytochrome P450 1A1 activity, critical for execution of antitumour activity, and protein expression are powerfully induced. 2-(4-Amino-3-methylphenyl)benzothiazole-derived covalent binding to cytochrome P450 1A1 is reduced by glutathione, suggesting 1A1-dependent production of a reactive electrophilic species. In vitro, 2-(4-aminophenyl)benzothiazole-generated DNA adducts form in sensitive tumour cells only. At concentrations >100 nM, adducts were detected in DNA of MCF-7 cells treated with 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203). 5F 203 (1 microM) led to the formation of one major and a number of minor adducts. However, treatment of cells with 10 microM 5F 203 resulted in the emergence of a new dominant adduct. Adducts accumulated steadily within DNA of MCF-7 cells exposed to 1 microM 5F 203 between 2 and 24 h. Concentrations of the lysylamide prodrug of 5F 203 (Phortress) > or = 100 nM generated adducts in the DNA of sensitive MCF-7 and IGROV-1 ovarian cells. At 1 microM, one major Phortress-derived DNA adduct was detected in these two sensitive phenotypes; 10 microM Phortress led to the emergence of an additional major adduct detected in the DNA of MCF-7 cells. Inherently resistant MDA-MB-435 breast carcinoma cells incurred no DNA damage upon exposure to Phortress (< or = 10 microM, 24 h). In vivo, DNA adducts accumulated within sensitive ovarian IGROV-1 and breast MCF-7 xenografts 24 h after treatment of mice with Phortress (20 mg kg(-1)). Moreover, Phortress-derived DNA adduct generation distinguished sensitive MCF-7 tumours from inherently resistant MDA-MB-435 xenografts implanted in opposite flanks of the same mouse.
DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells.[Pubmed:12592376]
Br J Cancer. 2003 Feb 24;88(4):599-605.
The fluorinated benzothiazole analogue 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is a novel agent with potent and selective antitumour properties and, in the form of its L-lysylamide prodrug Phortress (NSC 710305), is a current candidate for early phase clinical studies. Previous findings have indicated that cytochrome P450 1A1 (CYP1A1) may play a role in the antitumour activity of molecules in the benzothiazole series including the nonfluorinated parent compound 2-(4-amino-3-methylphenyl)benzothiazole (DF 203, NSC 674495) (Kashiyama et al, 1999; Chua et al, 2000; Loaiza-Perez et al, 2002). In this study, we assessed and verified that a fully functional aryl hydrocarbon receptor (AhR) signalling pathway is a necessary requisite for the induction of efficient cytotoxicity by 5F 203 in MCF-7 wild-type sensitive cells. Drug exposure caused MCF-7 sensitive cells to arrest in G(1) and S phase, and induced DNA adduct formation, in contrast to AhR-deficient AH(R100) variant MCF-7 cells. In sensitive MCF-7 cells, induction of CYP1A1 and CYP1B1 transcription (measured by luciferase reporter assay and real-time reverse transcriptase-polymerase chain reaction (RT-PCR)), and 7-ethoxyresorufin-O-deethylase (EROD) activity was demonstrated, following treatment with 5F 203. In contrast, in resistant AH(R100) cells, drug treatment did not affect CYP1A1 and CYP1B1 transcription and EROD activity. Furthermore, AH(R100) cells failed to produce either protein/DNA complexes on the xenobiotic responsive element (XRE) sequence of CYP1A1 promoter (measured by electrophoretic mobility shift assay) or DNA adducts. The data confirm that activation of the AhR signalling pathway is an important feature of the antitumour activity of 5F 203.


