PanaxytriolCAS# 87005-03-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 87005-03-6 | SDF | Download SDF |
| PubChem ID | 10540577 | Appearance | Powder |
| Formula | C17H26O3 | M.Wt | 278.4 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
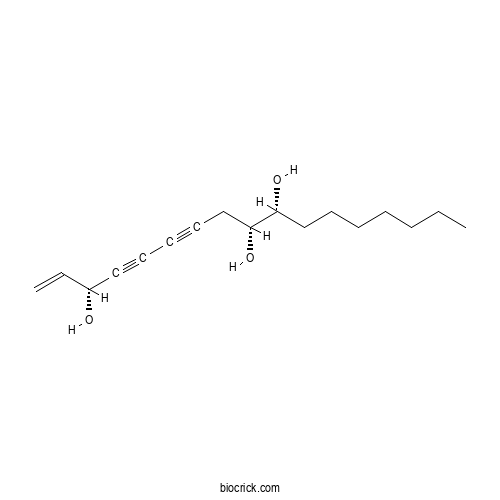
| Chemical Name | (3R,9R,10R)-heptadec-1-en-4,6-diyne-3,9,10-triol | ||
| SMILES | CCCCCCCC(C(CC#CC#CC(C=C)O)O)O | ||
| Standard InChIKey | RDIMTXDFGHNINN-BRWVUGGUSA-N | ||
| Standard InChI | InChI=1S/C17H26O3/c1-3-5-6-7-10-13-16(19)17(20)14-11-8-9-12-15(18)4-2/h4,15-20H,2-3,5-7,10,13-14H2,1H3/t15-,16-,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Panaxytriol Dilution Calculator

Panaxytriol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.592 mL | 17.9598 mL | 35.9195 mL | 71.8391 mL | 89.7989 mL |
| 5 mM | 0.7184 mL | 3.592 mL | 7.1839 mL | 14.3678 mL | 17.9598 mL |
| 10 mM | 0.3592 mL | 1.796 mL | 3.592 mL | 7.1839 mL | 8.9799 mL |
| 50 mM | 0.0718 mL | 0.3592 mL | 0.7184 mL | 1.4368 mL | 1.796 mL |
| 100 mM | 0.0359 mL | 0.1796 mL | 0.3592 mL | 0.7184 mL | 0.898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-O-Vanilloylglucose
Catalog No.:BCX0129
CAS No.:68985-14-8
- 11-Deoxyalisol A
Catalog No.:BCX0128
CAS No.:155800-98-9
- Erythro-1',2'-dihydroxyasarone
Catalog No.:BCX0127
CAS No.:146830-05-9
- cis-5-O-Feruloylquinic acid
Catalog No.:BCX0126
CAS No.:87099-75-0
- 2-Phenylethyl 2-O-beta-D-glucopyranosyl-beta-D-glucopyranoside
Catalog No.:BCX0125
CAS No.:239795-38-1
- 25-Methoxyalisol F
Catalog No.:BCX0124
CAS No.:2221029-53-2
- Eugenyl O-beta-apiofuranosyl-(1''->6')-O-beta-glucopyranoside
Catalog No.:BCX0123
CAS No.:136083-96-0
- Quercetin 3-glucosyl-(1->2)-galactoside
Catalog No.:BCX0122
CAS No.:95043-15-5
- Borapetoside A
Catalog No.:BCX0121
CAS No.:100202-29-7
- 2-Methoxyphenyl-4-propylene-1-O-beta-apiofuranosyl-(1-6)-beta-glucopyranoside
Catalog No.:BCX0120
CAS No.:2476709-47-2
- Methyl obacunoate
Catalog No.:BCX0119
CAS No.:751-48-4
- Apigenin 7,4'-diglucoside
Catalog No.:BCX0118
CAS No.:31737-50-5
- beta-Oxoacteoside
Catalog No.:BCX0131
CAS No.:149507-92-6
- Jatairidoid B
Catalog No.:BCX0132
CAS No.:1393577-30-4
- Pyrolaside A
Catalog No.:BCX0133
CAS No.:868632-29-5
- (2R,4S)-3,4-Dihydro-4-hydroxy-2,7-dimethyl-1(2H)-naphthalenone
Catalog No.:BCX0134
CAS No.:1932291-56-9
- Pyrocallianthaside A
Catalog No.:BCX0135
CAS No.:1004783-55-4
- 3,3'-Dihydroxy-4',5,7-trimethoxyflavan
Catalog No.:BCX0136
CAS No.:97914-22-2
- Alisol P
Catalog No.:BCX0137
CAS No.:1005191-19-4
- Crocin 3
Catalog No.:BCX0138
CAS No.:55750-85-1
- Jatamanvaltrate N
Catalog No.:BCX0139
CAS No.:1395056-08-2
- Pyrolaside B
Catalog No.:BCX0140
CAS No.:868632-32-0
- Pipernonaline
Catalog No.:BCX0141
CAS No.:88660-10-0
- 4,7,9,9'-Tetrahydroxy-3,3'-dimethoxy-8,4'-oxyneolignan 7-O-beta-D-glucopyranoside
Catalog No.:BCX0142
CAS No.:182056-97-9
Panaxytriol upregulates CYP3A4 expression through the interaction between nuclear regulators and DNA response elements.[Pubmed:36948264]
J Ethnopharmacol. 2023 Jun 28;310:116398.
ETHNOPHARMACOLOGICAL RELEVANCE: Cytochrome P3A4 (CYP3A4) is a crucial drug-metabolizing enzyme, and its expression is regulated by the pregnane X receptor (PXR), constitutive androstane receptor (CAR), steroid receptor coactivator 1 (SRC-1), and acetyltransferase P300. Panaxytriol is a naturally derived active substance extracted from the roots of Panax ginseng C. A. Mey. which is widely used clinically. Our previous studies have shown that Panaxytriol induces CYP3A4 expression through PXR activation, which is antagonized by high CAR expression. However, the underlying mechanism remains unclear. AIM OF THE STUDY: This study aimed to investigate the mechanism of Panaxytriol in inducing CYP3A4 expression via interactions between nuclear regulators and DNA response elements. MATERIALS AND METHODS: Immunoprecipitation technique was used to assess the binding levels of PXR and CAR with the coactivators SRC-1 and P300 in HepG2 and Huh-7 cells. Furthermore, chromatin immunoprecipitation assay was used to investigate the PXR and CAR interaction with the CYP3A4 promoter response element ER-6/DR-3. RESULTS: The binding of PXR to SRC-1, P300, and the response elements ER-6 and DR-3 was improved with an increase in Panaxytriol concentration (10-80 muM), and the binding affinity was further enhanced upon CAR silencing. The binding of CAR to SRC-1 and the response elements ER-6 and DR-3 was significantly higher at 80 muM Panaxytriol, whereas no significant binding was observed between CAR and P300. CONCLUSION: Panaxytriol promoted the recruitment of PXR to SRC-1 and P300, binding to ER-6 and DR-3, and upregulating CYP3A4 expression. Furthermore, an interactive dialogue regulatory mechanism between PXR and CAR was observed.
Cytotoxic Properties of C(17) Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells.[Pubmed:36296616]
Molecules. 2022 Oct 18;27(20):7027.
Although C(17) polyacetylenes from Panax ginseng exhibit cytotoxic properties against various tumor cells, there have been few experiments on epithelial ovarian carcinoma cells. This study aimed to investigate the cytotoxic effects of C(17) polyacetylenes from P. ginseng against ovarian cancer cell lines. Four unreported (1-4) and fifteen known (5-19) C(17) polyacetylenes were obtained from the roots of P. ginseng using repeated chromatography (open column, MPLC, and preparative HPLC). The chemical structures of all the compounds were determined by analyzing their spectroscopic data (NMR, IR, and optical rotation) and HR-MS. The structures of new polyacetylenes were elucidated as (3S,8S,9R,10R)-(-)-heptadeca-9,10-epoxy-4,6-diyne-3,8-diyl diacetate (1), (3S,8S,9R,10R)-(-)-heptadeca-1-en-9,10-epoxy-4,6-diyne-3,8-diyl diacetate (2), (-)-haptadeca-9,10-epoxy-8-methoxy-4,6-diyne-3,11-diol (3), and (3R,9R,10R)-(+)-3-acetoxy-9,10-dihydroxyheptadeca-1-en-4,6-diyne (4), named ginsenoynes O, P, and Q, and 3-acetyl Panaxytriol, respectively. Subsequently, in vitro experiments on A2780 and SKOV3 human epithelial ovarian carcinoma cells were performed to assess the cytotoxic properties of the isolates. Among the isolates, panaquinquecol 4 (15) exhibited the most remarkable cytotoxic effects on both human ovarian cancer cells A2780 (IC(50) value of 7.60 muM) and SKOV3 (IC(50) value of 27.53 muM). Therefore, C(17) polyacetylenes derived from P. ginseng may warrant further investigation for their therapeutic potential in epithelial ovarian cancer.
Panaxytriol upregulates CYP3A4 expression based on the interaction of PXR, CAR, HSP90alpha, and RXRalpha.[Pubmed:35417848]
Phytomedicine. 2022 Jul;101:154097.
BACKGROUND: Cytochrome P450 3A4 (CYP3A4) is one of the most important drug-metabolizing enzymes in the human body, mainly existing in the liver, small intestine, and kidney. Panaxytriol is one of the key active components in red ginseng and Shenmai injection. Our previous study demonstrated that Panaxytriol regulates CYP3A4 expression mainly by activating pregnancy X receptor (PXR). At a high concentration of Panaxytriol (80 muM), the constitutive androstane receptor (CAR) is also involved in the upregulation of CYP3A4. PURPOSE: This study investigated how the cofactors heat shock protein 90 alpha (HSP90alpha) and retinoid X receptor alpha (RXRalpha) interact with PXR and CAR to participate in the regulation of CYP3A4 by Panaxytriol from the perspective of the PXR and CAR interaction. METHODS: The mRNA and protein expressions of PXR, CAR, CYP3A4, RXRalpha, and HSP90alpha in HepG2 cells and Huh-7 cells were detected by quantitative PCR and western blot analysis, respectively. The binding levels of PXR and CAR to RXRalpha and HSP90alpha were determined by co-immunoprecipitation analysis. The nuclear translocation of PXR and RXRalpha into HepG2 cells and human (hCAR)-silenced HepG2 cells were measured by immunofluorescence. RESULTS: In HepG2 cells and Huh-7 cells, Panaxytriol (10-80 muM) upregulated CYP3A4 expression in a concentration-dependent manner by decreasing PXR binding to HSP90alpha and increasing PXR binding to RXRalpha. When hCAR was silenced, Panaxytriol further enhanced CYP3A4 expression by strengthening PXR binding to RXRalpha, but it had no significant effect on the binding level of PXR and HSP90alpha. Additionally, at the high concentration of 80 muM Panaxytriol, CAR binding to HSP90alpha was weakened while binding to RXRalpha was enhanced. CONCLUSION: Panaxytriol can upregulate CYP3A4 expression by promoting PXR dissociation from HSP90alpha and enhancing PXR binding to RXRalpha in HepG2 cells and Huh-7 cells. At high concentrations of Panaxytriol, CAR also participates in the induction of CYP3A4 through a similar mechanism. However, in general, CAR antagonizes PXR binding to RXRalpha, thereby attenuating the upregulation of CYP3A4 by Panaxytriol.
Antifeedant and Antifungal Activities of Metabolites Isolated from the Coculture of Endophytic Fungus Aspergillus tubingensis S1120 with Red Ginseng.[Pubmed:34786852]
Chem Biodivers. 2022 Jan;19(1):e202100608.
A new globoscinic acid derivative, aspertubin A (1) along with four known compounds, were obtained from the co-culture of Aspergillus tubingensis S1120 with red ginseng. The chemical structures of compounds were characterized by using spectroscopic methods, the calculated and experimental electronic circular dichroism. Panaxytriol (2) from red ginseng, and asperic acid (4) showed significant antifeedant effect with the antifeedant rates of 75 % and 80 % at the concentrations of 50 mug/cm(2) . Monomeric carviolin (3) and asperazine (5) displayed weak attractant activity on silkworm. All compounds were assayed for antifungal activities against phytopathogens A. tubingensis, Nigrospora oryzae and Phoma herbarum and the results indicated that autotoxic aspertubin A (1) and Panaxytriol (2) possessed selective inhibition against A. tubingensis with MIC values at 8 mug/mL. The co-culture extract showed higher antifeedant and antifungal activities against P. herbarum than those of monoculture of A. tubingensis in ordinary medium. So the medicinal plant and endophyte showed synergistic effect on the plant disease resistance by active compounds from the coculture of A. tubingensis S1120 and red ginseng.
Real-time monitoring and effector screening of APE1 based on rGO assisted DNA nanoprobe.[Pubmed:34610334]
Anal Biochem. 2021 Nov 15;633:114394.
Human apurinic/pyrimidine endonuclease 1 (APE1) played a critical role in the occurrence, progress and prognosis of tumors through overexpression and subcellular localization. Thus, it has become an important target for enhancing the sensitivity of tumor cells to radiotherapy and chemotherapy. Therefore, detecting and imaging its intracellular activity is of great significance for inhibitor discovery, cancer diagnosis and therapy. In this work, using DNA-based nanoprobe, we developed a new method for monitor intracellular APE1 activity. The detecting system was consisted by single fluorophore labeled hairpin probe and reduced graphene oxide (rGO). The in vitro result showed that a liner response of the detection method ranged from 0.02 U/mL to 2 U/mL with a limit of detection of 0.02 U/mL. Furthermore, this strategy possessing high specificity was successfully applied for APE1-related inhibitor screening using intracellular fluorescence imaging. Panaxytriol, an effective inhibitor of APE1 activity, was screened from traditional Chinese medicine (TCM) and its effect on APE1 activity was monitored in real time in A549 cells. In summary, this sensitive and specific APE1 detection technology is expected to provide an assistance for APE1-related inhibitor screening and diseases diagnosis.
Panaxytriol Inhibits Lipopolysaccharide-Induced Microglia Activation in Brain Inflammation in Vivo.[Pubmed:34193685]
Biol Pharm Bull. 2021;44(7):1024-1028.
Brain inflammation is a pathological characteristic of neurodegenerative diseases. In this condition, excessively activated microglia elevate proinflammatory mediator levels. We previously reported that Panaxytriol inhibited lipopolysaccharide (LPS)-induced microglia activation in vitro. However, the effects of Panaxytriol on microglia activation in vivo require confirmation. In the present study, we found that Panaxytriol suppressed both microglia and astrocyte activation by injected LPS intracerebrally to mice with LPS-induced brain inflammation. Panaxytriol was more effective on microglia than astrocytes. Moreover, Panaxytriol tended to reduce LPS-induced spontaneous motor activity dysfunction. These results suggested that Panaxytriol could improve brain health by suppressing microglia activation in neurodegenerative diseases.
Inhibitory effect of panaxytriol on BV-2 microglial cell activation.[Pubmed:33602508]
J Pharmacol Sci. 2021 Mar;145(3):273-278.
Activated microglia induce brain inflammation and neuronal death. Panaxytriol, ((3R,9R,10R)-Heptadec-1-en-4,6-diyne-3,9,10-triol), is a component of Panax ginseng C. A. Meyer extracts and activates the Nrf2-ARE signaling pathway. However, little is known about its effects on activated microglia in the brain. In this study, we investigated the effect of Panaxytriol on lipopolysaccharide (LPS)-induced activated microglia in BV-2 cells. Panaxytriol suppressed LPS-induced NO production and inhibited the increase in iNOS protein expression in BV-2 cells. Besides, Panaxytriol inhibited the mRNA expression of proinflammatory cytokines such as TNF-alpha, IL-1beta, and IL-6. The inhibitory effect of Panaxytriol on microglia activation did not affect the Nrf2-ARE pathway and the MAPK pathway. However, Panaxytriol suppressed LPS-induced NF-kappaB nuclear translocation. These results suggest that Panaxytriol inhibits the LPS-induced activation of microglia via the inhibition of NF-kappaB signaling pathway.
Biotransformation of natural polyacetylene in red ginseng by Chaetomium globosum.[Pubmed:33192119]
J Ginseng Res. 2020 Nov;44(6):770-774.
BACKGROUND: Fermentation has been shown to improve the biological properties of plants and herbs. Specifically, fermentation causes decomposition and/or biotransformation of active metabolites into high-value products. Polyacetylenes are a class of polyketides with a pleiotropic profile of bioactivity. METHODS: Column chromatography was used to isolate compounds, and extensive NMR experiments were used to determine their structures. The transformation of polyacetylene in red ginseng (RG) and the production of cazaldehyde B induced by the extract of RG were identified by TLC and HPLC analyses. RESULTS: A new metabolite was isolated from RG fermented by Chaetomium globosum, and this new metabolite can be obtained by the biotransformation of polyacetylene in RG. Panaxytriol was found to exhibit the highest antifungal activity against C. globosum compared with other major ingredients in RG. The fungus C. globosum cultured in RG extract can metabolize Panaxytriol to Metabolite A to survive, with no antifungal activity against itself. Metabolites A and B showed obvious inhibition against NO production, with ratios of 42.75 +/- 1.60 and 63.95 +/- 1.45% at 50 muM, respectively. A higher inhibitory rate on NO production was observed for Metabolite B than for a positive drug. CONCLUSION: Metabolite A is a rare example of natural polyacetylene biotransformation by microbial fermentation. This biotransformation only occurred in fermented RG. The extract of RG also stimulated the production of a new natural product, cazaldehyde B, from C. globosum. The lactone in Metabolite A can decrease the cytotoxicity, which was deemed to be the intrinsic activity of polyacetylene in ginseng.
Inducing Intermediates in Biotransformation of Natural Polyacetylene and A Novel Spiro-gamma-Lactone from Red Ginseng by Solid Co-Culture of Two Gut Chaetomium globosum and The Potential Bioactivity Modification by Oxidative Metabolism.[Pubmed:32182681]
Molecules. 2020 Mar 8;25(5):1216.
The omega-hydroxyl-Panaxytriol (1) and omega-hydroxyl-dihydroPanaxytriol (2)-are rare examples of polyacetylene metabolism by microbial transformation, and these new metabolites (1, 2) from fermented red ginseng (FRG) by solid co-culture induction of two Chaetomium globosum should be the intermediates of biotransformation of panaxylactone (metabolite A). The metabolic pathway of panaxylactone was also exhibited. The ingredients of red ginseng (RG) also induced the production of rare 6/5/5 tricyclic ring spiro-gamma-lactone skeleton (3). The omega-hydroxylation of new intermediates (1, 2) decreases cytotoxicity and antifungal activity against C. globosum compared with that of its bioprecursor Panaxytriol. Additionally, compounds 1 and 2 indicated obvious inhibition against nitric oxide (NO) production, with ratios of 44.80 +/- 1.37 and 23.10 +/- 1.00% at 50 muM. 1 has an equivalent inhibition of NO production compared with the positive drug. So, the microbial biotransformation that occurred in FRG fermented by gut C. globosum can change the original bioactivity of polyacetylene, which gave a basis about the metabolic modification of red ginseng by intestinal fungus fermentation.
Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng.[Pubmed:31885119]
Phytother Res. 2020 Jun;34(6):1226-1236.
Asian ginseng (Panax ginseng C.A. Meyer), American ginseng (Panax quinquefolius) and notoginseng (Panax notoginseng) are the three most commonly used ginseng botanicals in the world. With the increasing interests on antimicrobial properties of plants, the antimicrobial activities of ginseng species have been investigated by a number of researchers worldwide. This overview interprets our present knowledge of the antimicrobial activities of the three ginseng species and some of their bioactive components against pathogenic bacteria (Pseudomonas aeruginosa, Helicobacter pylori, Staphylococcus aureus, Escherichia coli, Propionibacterium acnes, et al.) and fungi (Candida albicans, Fusarium oxysporum, et al). Ginsenosides, polysaccharides, essential oil, proteins, and Panaxytriol are all might responsible for the antimicrobial activities of ginseng. The antimicrobial mechanisms of ginseng components could be summarized to the following points: (a) inhibit the microbial motility and quorum-sensing ability; (b) affect the formation of biofilms and destroy the mature biofilms, which can weaken the infection ability of the microbes; (c) perturb membrane lipid bilayers, thus causing the formation of pores, leakages of cell constituents and eventually cell death; (d) stimulate of the immune system and attenuate microbes induced apoptosis, inflammation, and DNA damages, which can protect or help the host fight against microbial infections; and (e) inhibit the efflux of antibiotics that can descend the drug resistance of the microbial. The collected information might facilitate and guide further studies needed to optimize the use of ginseng and their components to improve microbial food safety and prevent or treat animal and human infections.
Effect of panaxytriol on cytochrome P450 3A4 via the pregnane X receptor regulatory pathway.[Pubmed:30653754]
Phytother Res. 2019 Apr;33(4):968-975.
Panaxytriol (PXT) is one of the major effective components of red ginseng and Shenmai injection. The present study aimed to explore the effect of PXT on cytochrome P450 3A4 (CYP3A4) based on the pregnane X receptor (PXR)-CYP3A4 regulatory pathway in HepG2 cells and hPXR-overexpressing HepG2 cells treated with PXT for different time periods using quantitative polymerase chain reaction, Western blot, and dual-luciferase reporter gene assays. PXT could upregulate the levels of PXR and CYP3A4 mRNA in HepG2 cells treated with PXT for 1 hr, with no impact on the expression of their protein levels. The expression levels of both PXR and CYP3A4 mRNA and protein in HepG2 cells treated with PXT for 24 hr increased in a concentration-dependent manner. The effects of PXT on the expression of PXR and CYP3A4 mRNA and protein in hPXR-overexpressing HepG2 cells were similar to those in HepG2 cells. Moreover, the influence trend of PXT on CYP3A4 was consistent with that of PXR in HepG2 cells and hPXR-overexpressing HepG2 cells. The dual-luciferase reporter gene assay in HepG2 cells further demonstrated that PXT treatment for specific time periods could significantly induce the expression of CYP3A4 mediated by the PXR regulatory pathway.
Constitutive androstane receptor weakens the induction of panaxytriol on CYP3A4 by repressing the activation of pregnane X receptor.[Pubmed:30414935]
Biochem Pharmacol. 2019 Jan;159:32-39.
Nuclear receptors pregnane X receptor (PXR; NR1I2) and constitutive androstane receptor (CAR; NR1I3) play a vital role in regulating CYP3A4. Our previous studies have demonstrated that Panaxytriol (PXT) upregulates the expression of CYP3A4 via the PXR regulatory pathway. This study aimed to explore how CAR mediates the regulation of CYP3A4 in the presence of PXT using HepG2 cell, hCAR-overexpressing HepG2 cell and hCAR-silenced HepG2 cell models. In HepG2 cells, PXT induced the expression of CYP3A4 in a concentration-dependent manner (10-80 muM) and the high concentration of PXT (80 muM) upregulated the expression of CAR. The concentrations of PXT (10-40 muM) had no impact on the expression of CAR, but could significantly induce the expression of CYP2B6 target gene by activating CAR. The dual-luciferase reporter gene assay also showed that CAR-mediated CYP3A4 luciferase activity can be promoted by 80 muM of PXT (1.54-fold), while 5, 10, 20, and 40 muM of PXT had no influence on CAR-mediated CYP3A4 luciferase activity. In hCAR-overexpressing HepG2 cells, PXT concentrations (10-40 muM) that significantly induced PXR and CYP3A4 in HepG2 cells had no impact on the expression of CYP3A4, CAR and PXR, whereas a high concentration of PXT (80 microM) could weakly induce the mRNA and protein levels of CAR and CYP3A4. Moreover, the expression of PXR and CYP3A4 in hCAR-silenced HepG2 cells was markedly elevated compared with the blank control or with normal HepG2 cells treated with 10-80 muM of PXT. In conclusion, CAR significantly weakens the ability of PXT to induce CYP3A4 expression by repressing the activation of PXR. There may be a cross-talk mechanism between PXR and CAR on the regulation of CYP3A4 in the presence of PXT. Additionally, a high concentration of PXT (80 muM) induced CYP3A4 via the CAR regulatory pathway.
Inhibitory Effects of Polyacetylene Compounds from Panax ginseng on Neurotrophin Receptor-Mediated Hair Growth.[Pubmed:28966252]
Biol Pharm Bull. 2017;40(10):1784-1788.
Neurotrophins play an important role in the control of the hair growth cycle. Therefore, neurotrophin receptor antagonists have therapeutic potential for the treatment of hair growth disorders. In this study, we investigated the inhibitory effect of Panax ginseng, a medicinal plant commonly used to treat alopecia, on the binding of neurotrophins to their receptors. In addition, we isolated and characterized the bioactive compounds of P. ginseng extracts. P. ginseng hexane extracts strongly inhibited brain-derived neurotrophic factor (BDNF)-TrkB and beta-nerve growth factor (beta-NGF)-p75 neurotrophin receptor (p75NTR) binding. Furthermore, we identified the following 6 polyacetylene compounds as the bioactive components in P. ginseng hexane extract: panaxynol (1), panaxydol (2), panaxydol chlorohydrin (3), 1,8-heptadecadiene-4,6-diyne-3,10-diol (4), Panaxytriol (5), and dihydropanaxacol (6). In particular, compounds 4, 5, and 6 significantly inhibited BDNF-TrkB binding in a dose-dependent manner. To identify the structural component mediating the inhibitory effect, we investigated the effects of the hydroxyl moiety in these compounds. We found that the inhibitory effect of Panaxytriol (5) was strong, whereas the inhibitory effect of Ac-Panaxytriol (7) was relatively weak. Our findings suggest that P. ginseng-derived polyacetylenes with a hydroxyl moiety might provide therapeutic benefits to patients with hair growth disorders such as alopecia by inhibiting the binding of neurotrophins to their receptors. Although saponins have been proposed to be the primary mediators of the effects of P. ginseng on hair growth, this study revealed that polyacetylene compounds exert similar effects.
Polyacetylenic Oleanane-Type Triterpene Saponins from the Roots of Panax japonicus.[Pubmed:28006911]
J Nat Prod. 2016 Dec 23;79(12):3079-3085.
Three new polyacetylenic oleanane-type triterpenoids, baisanqisaponins A-C (1-3), and one new oleanane-type triterpenoid, chikusetsusaponin-V ethyl ester (4), together with 19 known compounds (5-23), were isolated from the roots of Panax japonicus. The structures were elucidated on the basis of spectroscopic analyses and chemical methods. Compounds 1-3 feature a rare Panaxytriol group containing a polyacetylene on the saponin skeleton. Neuroprotective activity was evaluated for compounds 1-17, and angiotensin II-induced vascular smooth muscle cell proliferation inhibition was tested for compounds 5-7 and 10-12.
Cytotoxicity of lipid-soluble ginseng extracts is attenuated by plasma membrane redox enzyme NQO1 through maintaining redox homeostasis and delaying apoptosis in human neuroblastoma cells.[Pubmed:27704336]
Arch Pharm Res. 2016 Oct;39(10):1339-1348.
Lipid-soluble ginseng extracts (LSGE) is known to inhibit many types of cancer cells through arresting cell cycle and inducing apoptosis. Usually, normal cells are can also be damaged by anti-tumor reagents. The plasma membrane redox system (PMRS) is enhanced to compensate mitochondrial dysfunction and impaired energy metabolism. NADH-quinone oxidoreductase 1 (NQO1), a plasma membrane redox enzyme, is known to be induced by Panaxytriol, one of components of lipid-soluble ginseng extracts (LSGE). The objective of this study was determine the mechanisms of NQO1 involved in neuroprotection in response to cytotoxicity induced by LSGE. Exposure of control SH-SY5Y cells to LSGE resulted in dramatic loss of cell viability in a dose-dependent manner. The loss of cell viability was significantly recovered in cells transfected with NQO1. LSGE-induced cell death occurred through apoptosis such as cell shrinkage, chromatin condensation and cleavage of poly (ADP-ribose) polymerase. These apoptotic features were significantly attenuated by overexpression of NQO1. Levels of oxidative/nitrative damage were highly elevated by LSGE in a dose-dependent manner. However, these elevated levels were greatly reduced by overexpression of NQO1. In addition, overexpression of NQO1 attenuated the decrease in mitochondrial complex I activity caused by LSGE. Taken together, these findings suggest that overexpressed NQO1 can protect cells against LSGE-induced cytotoxicity through lowering oxidative/nitrative damage and delaying apoptosis, supporting that stimulation of NQO1 activity could be a therapeutic targets in neurodegeration.


