NorlichexanthoneCAS# 20716-98-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20716-98-7 | SDF | Download SDF |
| PubChem ID | 5281657 | Appearance | Yellow powder |
| Formula | C14H10O5 | M.Wt | 258.2 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
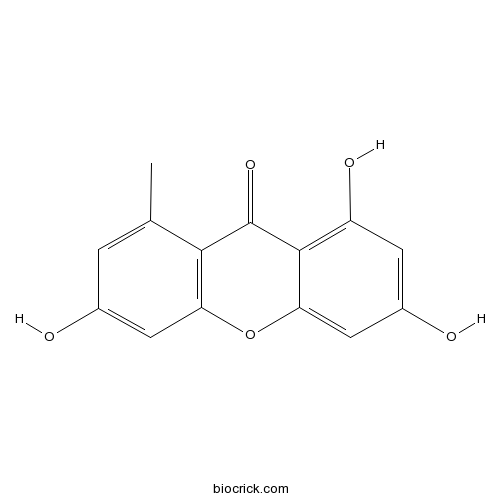
| Chemical Name | 1,3,6-trihydroxy-8-methylxanthen-9-one | ||
| SMILES | CC1=CC(=CC2=C1C(=O)C3=C(C=C(C=C3O2)O)O)O | ||
| Standard InChIKey | AQZHBCDRWFMXIN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O5/c1-6-2-7(15)4-10-12(6)14(18)13-9(17)3-8(16)5-11(13)19-10/h2-5,15-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Norlichexanthone can promote the secretion and expression of adiponectin in cultured ST-13 adipocytes, it has the potential to treat and/or prevent lifestyle-related diseases, including metabolic syndrome, type 2 diabetes, atherosclerosis and cardiovascular diseases. 2. Norlichexanthone has antibacterial activity, it shows strong activity against Bacillus subtilis with IC50 in the range of 1-5uM, it also significantly inhibits the growth of methicillin-resistant Staphylococcus aureus with IC50 of 20.95±1.56uM. 3. Norlichexanthone produces significant chemosuppression of parasitaemia, it has antimalarial activity. |
| Targets | PPAR | Antifection |

Norlichexanthone Dilution Calculator

Norlichexanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.873 mL | 19.3648 mL | 38.7297 mL | 77.4593 mL | 96.8242 mL |
| 5 mM | 0.7746 mL | 3.873 mL | 7.7459 mL | 15.4919 mL | 19.3648 mL |
| 10 mM | 0.3873 mL | 1.9365 mL | 3.873 mL | 7.7459 mL | 9.6824 mL |
| 50 mM | 0.0775 mL | 0.3873 mL | 0.7746 mL | 1.5492 mL | 1.9365 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3873 mL | 0.7746 mL | 0.9682 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neosperidin dihydrochalcone
Catalog No.:BCN5016
CAS No.:20702-77-6
- BD 1063 dihydrochloride
Catalog No.:BCC6832
CAS No.:206996-13-6
- Methysticin
Catalog No.:BCN2306
CAS No.:20697-20-5
- Tariquidar
Catalog No.:BCC3625
CAS No.:206873-63-4
- Vincristine sulfate
Catalog No.:BCN2542
CAS No.:2068-78-2
- 3,7-Di-O-methylquercetin
Catalog No.:BCN6486
CAS No.:2068-02-2
- Pedatisectine F
Catalog No.:BCN4902
CAS No.:206757-32-6
- Ecliptasaponin D
Catalog No.:BCN2760
CAS No.:206756-04-9
- Cannabichromene
Catalog No.:BCN4901
CAS No.:20675-51-8
- Pasiniazid
Catalog No.:BCC3835
CAS No.:2066-89-9
- 7-O-Methylporiol
Catalog No.:BCN3948
CAS No.:206560-99-8
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
- Fustin
Catalog No.:BCN4904
CAS No.:20725-03-5
- TB 21007
Catalog No.:BCC7510
CAS No.:207306-50-1
- Methyl sinapate
Catalog No.:BCN4675
CAS No.:20733-94-2
- 1,8-Bis(dimethylamino)naphtalene
Catalog No.:BCC8429
CAS No.:20734-58-1
- Saikosaponin C
Catalog No.:BCN1087
CAS No.:20736-08-7
- Saikosaponin A
Catalog No.:BCN1086
CAS No.:20736-09-8
- Dihydrosinapylalcohol
Catalog No.:BCN6542
CAS No.:20736-25-8
- 3-Bromocytisine
Catalog No.:BCC7705
CAS No.:207390-14-5
- MR 16728 hydrochloride
Catalog No.:BCC6684
CAS No.:207403-36-9
- Arteannuin L
Catalog No.:BCN4905
CAS No.:207446-89-7
- Arteannuin M
Catalog No.:BCN4906
CAS No.:207446-90-0
- Arteannuin N
Catalog No.:BCN4907
CAS No.:207446-92-2
In-vivo antimalarial activity of some oxygenated xanthones.[Pubmed:14613627]
Ann Trop Med Parasitol. 2003 Oct;97(7):683-8.
A series of oxygenated xanthones was prepared so that the antimalarial activity of each compound could be evaluated in vivo, using 4-day suppressive assays against Plasmodium berghei ANKA in BALB/c mice. When given in a dose of 20 mg/kg.day for 4 days, most of the compounds produced significant chemosuppression of parasitaemia. The most active compound was 1,3,6,8-tetrahydroxyxanthone, which reduced the percentage of erythrocytes infected by 70.5%, followed by Norlichexanthone (44.3%) and its isomer, 1,3,8-trihydroxy-6-methylxanthone (37.0%). Whereas di-C-allyl-dihydroxyxanthone showed lower but still notable activity (33.4%), 1,3-dihydroxyxanthone was much less active (15.1%). This appears to be the first demonstration of the antimalarial activity of some hydroxyxanthones in vivo.
Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp.[Pubmed:22061662]
Fitoterapia. 2012 Jan;83(1):209-14.
Two new polyketides, 7-hydroxy-3, 5-dimethyl-isochromen-1-one (1) and 6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione (2), along with eleven known compounds, 5'-methoxy-6-methyl-biphenyl-3,4,3'-triol (3), 7-hydroxy-3-(2-hydroxy-propyl)-5-methyl-isochromen-1-one (4), rubralactone (5), isoaltenuene (6), altenuene (7), dihydroaltenuenes A (8), altenusin (9), alterlactone (10), 6-O-methylNorlichexanthone (11), Norlichexanthone (12), and griseoxanthone C (13) were isolated from the culture of the endolichenic fungus Ulocladium sp. Compound 2 was obtained as a racemate with an unprecedented chemical skeleton. The NMR data assignments for 3 and 4 were achieved for the first time. Compounds 1-13 were screened for their antimicrobial and radical scavenging activities. Compound 1 showed some antifungal activity against Candida albicans SC 5314 with IC(50) of 97.93 +/- 1.12 muM. Compounds 11-13 showed strong activity against Bacillus subtilis with IC(50) in the range of 1-5 muM. Compound 12 significantly inhibited the growth of methicillin-resistant Staphylococcus aureus with IC(50) of 20.95 +/- 1.56 muM. Compounds 9 and 10 showed strong radical scavenging activity in comparison with vitamin C. The plausible biosynthetic pathways for compounds 1, 2, and 4-8 were discussed.
Norlichexanthone isolated from fungus P16 promotes the secretion and expression of adiponectin in cultured ST-13 adipocytes.[Pubmed:21568875]
Med Chem. 2011 Jul;7(4):250-6.
Adiponectin, an adipose-derived protein, shows insulin-sensitizing, anti-diabetic and anti-atherogenic activities, which implies that the protein represents a potential target to improve lifestyle-related diseases like type 2 diabetes. Based on our hypothesis that agents that cause adipocyte differentiation could also act as adiponectin secretion enhancers, we screened butanol extracts of 96 fungus culture extracts for their differentiation-inducing activity in ST-13 preadipocytes. We found that the butanol extract of a fungus P16 culture extract possessed such an activity, and isolated Norlichexanthone as an active compound through activity-guided fractionation. Oil red O staining showed that Norlichexanthone induced adipogenesis in ST-13 cells. Its differentiation-inducing activity was supported by the observation that Norlichexanthone dose-dependently increased the mRNA expression of fatty acid-binding protein and peroxisome proliferator activated receptor gamma (PPARgamma), markers of adipocyte differentiation. Western blot analysis demonstrated that the compound enhanced the secretion of adiponectin protein in a dose-dependent manner. An increase in mRNA expression of adiponectin was also observed in the Norlichexanthone-treated ST-13 cells. Actinomycin D treatment blocked the enhancement of adiponectin mRNA expression by Norlichexanthone, suggesting that it is the result of increased transcription. A luciferase reporter assay indicated that Norlichexanthone was unlikely to be an agonist of PPARgamma, implying that its action of mechanism might differ from those of thiazolidinediones which upregulate adiponectin expression via activation of PPARgamma. These findings suggest the possibility that Norlichexanthone has the potential to treat and/or prevent lifestyle-related diseases, including metabolic syndrome, type 2 diabetes, atherosclerosis and cardiovascular diseases.


