Noradrenaline bitartrate monohydrateEndogenous adrenergic hormone and neurotransmitter CAS# 108341-18-0 |
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- LY450108
Catalog No.:BCC1725
CAS No.:376594-67-1
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Aniracetam
Catalog No.:BCC4219
CAS No.:72432-10-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

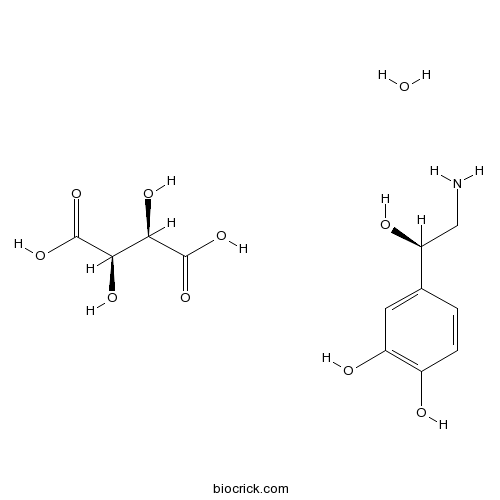
| Cas No. | 108341-18-0 | SDF | Download SDF |
| PubChem ID | 3047796 | Appearance | Powder |
| Formula | C12H19NO10 | M.Wt | 337.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Norepinephrine | ||
| Solubility | H2O : 50 mg/mL (148.24 mM; Need ultrasonic) DMSO : ≥ 30 mg/mL (88.95 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[(1R)-2-amino-1-hydroxyethyl]benzene-1,2-diol;(2R,3R)-2,3-dihydroxybutanedioic acid;hydrate | ||
| SMILES | C1=CC(=C(C=C1C(CN)O)O)O.C(C(C(=O)O)O)(C(=O)O)O.O | ||
| Standard InChIKey | LNBCGLZYLJMGKP-LUDZCAPTSA-N | ||
| Standard InChI | InChI=1S/C8H11NO3.C4H6O6.H2O/c9-4-8(12)5-1-2-6(10)7(11)3-5;5-1(3(7)8)2(6)4(9)10;/h1-3,8,10-12H,4,9H2;1-2,5-6H,(H,7,8)(H,9,10);1H2/t8-;1-,2-;/m01./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous adrenergic hormone and neurotransmitter. A classical stress hormone; underlies the fight or flight response, along with adrenaline. Increases heart rate. Vasoconstrictor. |

Noradrenaline bitartrate monohydrate Dilution Calculator

Noradrenaline bitartrate monohydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9649 mL | 14.8245 mL | 29.649 mL | 59.2979 mL | 74.1224 mL |
| 5 mM | 0.593 mL | 2.9649 mL | 5.9298 mL | 11.8596 mL | 14.8245 mL |
| 10 mM | 0.2965 mL | 1.4824 mL | 2.9649 mL | 5.9298 mL | 7.4122 mL |
| 50 mM | 0.0593 mL | 0.2965 mL | 0.593 mL | 1.186 mL | 1.4824 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2965 mL | 0.593 mL | 0.7412 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Noradrenaline bitartrate monohydrate
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
- Fmoc-D-Asn-OH
Catalog No.:BCC3083
CAS No.:108321-39-7
- 1,7-Bis(4-hydroxyphenyl)hept-1-en-3-one
Catalog No.:BCN1629
CAS No.:1083200-79-6
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN1630
CAS No.:1083195-05-4
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- A 987306
Catalog No.:BCC7732
CAS No.:1082954-71-9
- LY2584702
Catalog No.:BCC6369
CAS No.:1082949-67-4
- PF-04447943
Catalog No.:BCC1850
CAS No.:1082744-20-4
- PDE-9 inhibitor
Catalog No.:BCC1842
CAS No.:1082743-70-1
- TC-S 7005
Catalog No.:BCC6189
CAS No.:1082739-92-1
- TUG 424
Catalog No.:BCC7776
CAS No.:1082058-99-8
- Fuligorubin A
Catalog No.:BCN1837
CAS No.:108343-55-1
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
- Ilexsaponin A
Catalog No.:BCN7867
CAS No.:108524-93-2
- Ilexgenin A
Catalog No.:BCC9233
CAS No.:108524-94-3
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- Lumichrome
Catalog No.:BCN7083
CAS No.:1086-80-2
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
Sibutramine, a serotonin-norepinephrine reuptake inhibitor, causes fibrosis in rats.[Pubmed:26070021]
Environ Toxicol Pharmacol. 2015 Jul;40(1):71-6.
Sibutramine hydrochloride monohydrate is a weight loss agent indicated for the treatment of obesity. Although it has been banned from most markets, studies are still relevant as it is often a hidden ingredient in herbal and over the counter slimming products. Sibutramine induces liver fibrosis with steatosis in female Sprague-Dawley rats fed a high-energy diet without significant weight gain. In this study, using the same animal model, the effect of Sibutramine on lung morphology was investigated using histological evaluation of the terminal bronchiole and transmission electron microscopy evaluation of the respiratory tissue. From these results Sibutramine was found to induce lung fibrosis in Sprague-Dawley rats as increased collagen synthesis, mast cell accumulation and aggregates of Bronchus Associated Lymphoid Tissue (BALT) in the terminal bronchiole as well as increased collagen deposition in the respiratory tissue was seen.
An Open-Label Pilot Study of Combined Augmentation With Creatine Monohydrate and 5-Hydroxytryptophan for Selective Serotonin Reuptake Inhibitor- or Serotonin-Norepinephrine Reuptake Inhibitor-Resistant Depression in Adult Women.[Pubmed:28787372]
J Clin Psychopharmacol. 2017 Oct;37(5):578-583.
PURPOSE: Many women with major depressive disorder (MDD) respond inadequately to standard treatments. Augmentation of conventional antidepressants with creatine monohydrate and 5-hydroxytryptophan (5-HTP) could correct deficits in serotonin production and brain bioenergetics associated with depression in women, yielding synergistic benefit. We describe an open-label study of 5-HTP and creatine augmentation in women with MDD who had failed selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) monotherapy. METHODS: Fifteen women who were adequately adherent to an SSRI or SNRI and currently experiencing MDD, with a 17-item Hamilton Depression Rating Scale (HAM-D) score of 16 or higher, were treated with 5 g of creatine monohydrate daily and 100 mg of 5-HTP twice daily for 8 weeks, with 4 weeks of posttreatment follow-up. The primary outcome was change in mean HAM-D scores. RESULTS: Mean HAM-D scores declined from 18.9 (SD, 2.5) at pretreatment visits to 7.5 (SD, 4.4) (P < 0.00001), a decrease of 60%. Participants did not experience any serious treatment-related adverse events. CONCLUSIONS: Combination treatment with creatine and 5-HTP may represent an effective augmentation strategy for women with SSRI- or SNRI-resistant depression. Given the limitations of this small, open-label trial, future study in randomized, placebo-controlled trials is warranted.
Norepinephrine responses in rat renal and femoral veins are reinforced by vasoconstrictor prostanoids.[Pubmed:26141930]
Vascul Pharmacol. 2015 Sep;72:93-100.
Norepinephrine (NE) responses are larger in renal and femoral veins compared to phenylephrine (PE). These differences may be due to the subtypes of adrenoceptor involved in these responses or to the involvement of local modulatory mechanisms. Therefore, the present study investigated in organ bath the adrenoceptor subtypes involved in the NE and PE responses in both renal and femoral veins as well as the influence of local mechanisms related to NO and to prostanoids upon these responses. The obtained data showed that the NE responses in these veins were not significantly modified by the selective inhibition of beta1 or beta2-adrenoceptors as well as AT1 or AT2 receptors. However, yohimbine reduced the NE Rmax in renal veins and, in parallel, right shifted the NE concentration-response curves in femoral veins. In both veins, prazosin reduced the NE Rmax and the clonidine induced a measurable contraction. The endothelium removal attenuated the NE responses in femoral veins, thereby abolishing the differences of NE and PE responses. Furthermore, the NE responses in renal and femoral veins were attenuated by indomethacin, which suppressed the statistical difference in relation to the PE response. In conclusion, a synergism between alpha1- and alpha2-adrenoceptors is essential to assure full NE contractile responses in both renal and femoral veins. Thus, by acting simultaneously in these adrenoceptors, NE induces more pronounced contractile responses, in comparison to PE, not only in renal but also in femoral veins. Moreover, this pronounced NE response in both renal and femoral veins appears to involve endothelium-derived vasoconstrictor prostanoids.
Antidepressant-like effects of Brassica juncea L. leaves in diabetic rodents.[Pubmed:24956892]
Indian J Exp Biol. 2014 Jun;52(6):613-22.
The objective of the study was to evaluate for antidepressant like activity of a methanolic extract of B. juncea leaves (BJ 100, 200, and 400 mg/kg/day, po), and Imipramine (15 mg/kg/day, po) in alloxan monohydrate (120 mg/kg, ip) induced diabetic and nondiabetic rodents, using behavioural despair, learned helplessness, and tail suspension tests for antidepressants and locomotor activity test for quantifying the behavioural effects of treatments. In addition, effects of BJ treatments on brain levels of norepinephrine, serotonin and dopamine were also estimated. Enhanced depressive states, and motility were observed in diabetic animals. Antidepressant and motor function depressing effects of BJ were apparent in all behavioural tests in diabetic rats and mice only. Decreased contents of dopamine, norepinephrine and serotonin in brain of diabetic rats were also dose dependently compensated by repeated daily BJ treatments. However, brain dopamine level of BJ treated normal rats was higher than that in control nondiabetic. The results suggest that BJ could be a nutritional alternative for combating exaggerated depression commonly associated with diabetes.
Norepinephrine: the next therapeutics frontier for Parkinson's disease.[Pubmed:23211006]
Transl Neurodegener. 2012 Jan 13;1(1):4.
Tissue concentrations of norepinephrine (NE) are markedly decreased in various regions of the Parkinson's disease (PD) brain. As in the substantia nigra pars compacta, neuronal dropout and Lewy bodies are prominent changes affecting the locus coeruleus, which is the source of ascending NErgic projections. Despite the major roles of NE throughout the brain, there has been only minimal exploration of pharmacological intervention with NErgic neurotransmission. Cognitive operations, "freezing" of gait, tremor, dyskinesia, REM sleep regulation, and other aspects of brain function are tied into signaling by NE, and there is also evidence that it may have a role in the neurodegenerative process itself. This article reviews the reported pharmacological experience in PD therapeutics.


