NeoeriocitrinCAS# 13241-32-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13241-32-2 | SDF | Download SDF |
| PubChem ID | 114627 | Appearance | White powder |
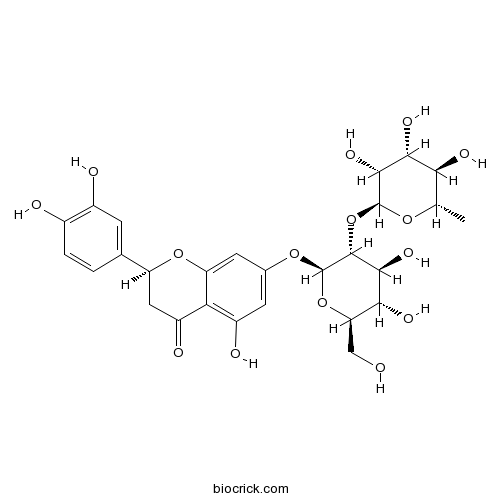
| Formula | C27H32O15 | M.Wt | 596.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Eriodictyol 7-neohesperidoside; 3',4',5,7-Tetrahydroxyflavanone 7-neohesperidoside | ||
| Solubility | Soluble in methanol; insoluble in water | ||
| Chemical Name | (2S)-7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5-hydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(C(OC2OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC(=C(C=C5)O)O)O)CO)O)O)O)O)O | ||
| Standard InChIKey | OBKKEZLIABHSGY-DOYQYKRZSA-N | ||
| Standard InChI | InChI=1S/C27H32O15/c1-9-20(33)22(35)24(37)26(38-9)42-25-23(36)21(34)18(8-28)41-27(25)39-11-5-14(31)19-15(32)7-16(40-17(19)6-11)10-2-3-12(29)13(30)4-10/h2-6,9,16,18,20-31,33-37H,7-8H2,1H3/t9-,16-,18+,20-,21+,22+,23-,24+,25+,26-,27+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Neoeriocitrin has antioxidant capacity, it could rescue the inhibition effect of cell differentiation induced by PD98059 to some degree.Neoeriocitrin may be a new promising candidate drug for treatment of osteoporosis. |
| Targets | LDL | Runx2 | COLI | OCN |
| In vitro | Comparison of neoeriocitrin and naringin on proliferation and osteogenic differentiation in MC3T3-E1.[Pubmed: 21741227]Phytomedicine. 2011 Aug 15;18(11):985-9.Naringin is considered the main effective compound of Drynaria Rhizome, which is used commonly in the treatment of osteoporosis in traditional Chinese medicine. However, we found Neoeriocitrin, a new compound isolated from Drynaria Rhizome, showed a better activity than naringin on proliferation and osteogenic differentiation in MC3T3-E1. Antioxidant activity of citrus limonoids, flavonoids, and coumarins.[Pubmed: 15769128 ]J Agric Food Chem. 2005 Mar 23;53(6):2009-14.
|

Neoeriocitrin Dilution Calculator

Neoeriocitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6764 mL | 8.3822 mL | 16.7645 mL | 33.5289 mL | 41.9111 mL |
| 5 mM | 0.3353 mL | 1.6764 mL | 3.3529 mL | 6.7058 mL | 8.3822 mL |
| 10 mM | 0.1676 mL | 0.8382 mL | 1.6764 mL | 3.3529 mL | 4.1911 mL |
| 50 mM | 0.0335 mL | 0.1676 mL | 0.3353 mL | 0.6706 mL | 0.8382 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1676 mL | 0.3353 mL | 0.4191 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chrysophanol 8-O-glucoside
Catalog No.:BCN6175
CAS No.:13241-28-6
- β-D-Glucopyranoside,(3β,6α,16β,20R,24S)-3-[(3,4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20, 24-epoxy-16,25-dihydroxy-9,19-cyclolanostan-6-yl
Catalog No.:BCC8370
CAS No.:1324005-51-7
- TC-G 1006
Catalog No.:BCC6277
CAS No.:1324003-64-6
- Boc-Gln(Trt)-OH
Catalog No.:BCC3384
CAS No.:132388-69-3
- Boc-Asn(Trt)-OH
Catalog No.:BCC3360
CAS No.:132388-68-2
- Fmoc-Gln(Trt)-OPfp
Catalog No.:BCC3486
CAS No.:132388-65-9
- Fmoc-Asn(Trt)-OPfp
Catalog No.:BCC3082
CAS No.:132388-64-8
- Z-Gln(Trt)-OH
Catalog No.:BCC2782
CAS No.:132388-60-4
- Fmoc-Asn(Trt)-OH
Catalog No.:BCC3081
CAS No.:132388-59-1
- H-Asn(Trt)-OH
Catalog No.:BCC2878
CAS No.:132388-58-0
- 10-Hydroxyneoline
Catalog No.:BCN6510
CAS No.:132362-42-6
- 5-Acetoxy-7-hydroxyflavone
Catalog No.:BCN6174
CAS No.:132351-58-7
- Neohesperidin
Catalog No.:BCN5915
CAS No.:13241-33-3
- Alosetron-d3 Hydrochloride
Catalog No.:BCC1345
CAS No.:1189919-71-8
- 2-TEDC
Catalog No.:BCC6735
CAS No.:132465-10-2
- (Z)-Pugnac
Catalog No.:BCC5333
CAS No.:132489-69-1
- Olanzapine
Catalog No.:BCC5042
CAS No.:132539-06-1
- 15-Demethylplumieride
Catalog No.:BCN6176
CAS No.:132586-69-7
- Bruceanic acid C
Catalog No.:BCN7999
CAS No.:132587-60-1
- CCMQ
Catalog No.:BCC6984
CAS No.:132623-44-0
- Otophylloside O
Catalog No.:BCN7337
CAS No.:1326583-08-7
- Senecionine N-oxide
Catalog No.:BCN2130
CAS No.:13268-67-2
- Fmoc-Tle-OH
Catalog No.:BCC2657
CAS No.:132684-60-7
- [Leu31,Pro34]-Neuropeptide Y (human, rat)
Catalog No.:BCC5722
CAS No.:132699-73-1
Comparison of neoeriocitrin and naringin on proliferation and osteogenic differentiation in MC3T3-E1.[Pubmed:21741227]
Phytomedicine. 2011 Aug 15;18(11):985-9.
Naringin is considered the main effective compound of Drynaria Rhizome, which is used commonly in the treatment of osteoporosis in traditional Chinese medicine. However, we found Neoeriocitrin, a new compound isolated from Drynaria Rhizome, showed a better activity than naringin on proliferation and osteogenic differentiation in MC3T3-E1. Both Neoeriocitrin and naringin exhibited the best effect on proliferation and osteogenic differentiation at concentration of 2mug/ml. Neoeriocitrin more significantly improved proliferation and alkaline phosphatase (ALP) activity as well as up-regulated Runx2, COLI and OCN expression by 56%, 37% and 14% respectively than naringin. Furthermore, Neoeriocitrin could rescue the inhibition effect of cell differentiation induced by PD98059 to some degree. Therefore, Neoeriocitrin may be a new promising candidate drug for treatment of osteoporosis.
Antioxidant activity of citrus limonoids, flavonoids, and coumarins.[Pubmed:15769128]
J Agric Food Chem. 2005 Mar 23;53(6):2009-14.
A variety of in vitro models such as beta-carotene-linoleic acid, 1,1-diphenyl-2-picryl hydrazyl (DPPH), superoxide, and hamster low-density lipoprotein (LDL) were used to measure the antioxidant activity of 11 citrus bioactive compounds. The compounds tested included two limonoids, limonin (Lim) and limonin 17-beta-D-glucopyranoside (LG); eight flavonoids, apigenin (Api), scutellarein (Scu), kaempferol (Kae), rutin trihydrate (Rut), neohesperidin (Neh), Neoeriocitrin (Nee), naringenin (Ngn), and naringin(Ng); and a coumarin (bergapten). The above compounds were tested at concentration of 10 microM in all four methods. It was found that Lim, LG, and Ber inhibited <7%, whereas Scu, Kae, and Rut inhibited 51.3%, 47.0%, and 44.4%, respectively, using the beta-carotene-linoleate model system. Lim, LG, Rut, Scu, Nee, and Kae showed 0.5% 0.25%, 32.2%, 18.3%, 17.2%, and 12.2%, respectively, free radical scavenging activity using the DPPH method. In the superoxide model, Lim, LG, and Ber inhibited the production of superoxide radicals by 2.5-10%, while the flavonoids such as Rut, Scu, Nee, and Neh inhibited superoxide formation by 64.1%, 52.1%, 48.3%, and 37.7%, respectively. However, LG did not inhibit LDL oxidation in the hamster LDL model. But, Lim and Ber offered some protection against LDL oxidation, increasing lag time to 345 min (3-fold) and 160 min (33% increase), respectively, while both Rut and Nee increased lag time to 2800 min (23-fold). Scu and Kae increased lag time to 2140 min (18-fold) and 1879 min (15.7-fold), respectively. In general, it seems that flavonoids, which contain a chromanol ring system, had stronger antioxidant activity as compared to limonoids and bergapten, which lack the hydroxy groups. The present study confirmed that several structural features were linked to the strong antioxidant activity of flavonoids. This is the first report on the antioxidant activity of limonin, limonin glucoside, and Neoeriocitrin.


