NeocnidilideCAS# 4567-33-3 |
- Sedanolide
Catalog No.:BCN8338
CAS No.:6415-59-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4567-33-3 | SDF | Download SDF |
| PubChem ID | 3083857 | Appearance | Powder |
| Formula | C12H18O2 | M.Wt | 194.27 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
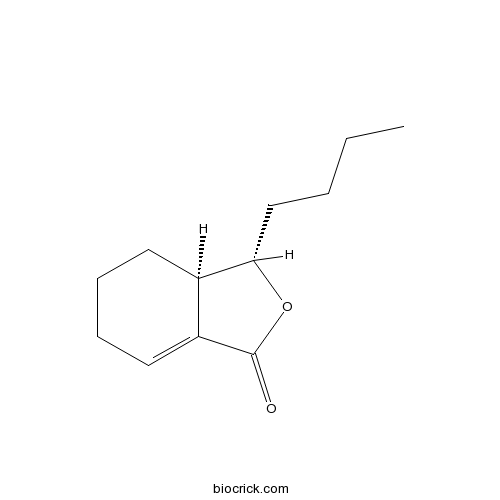
| Chemical Name | (3S,3aR)-3-butyl-3a,4,5,6-tetrahydro-3H-2-benzofuran-1-one | ||
| SMILES | CCCCC1C2CCCC=C2C(=O)O1 | ||
| Standard InChIKey | UPJFTVFLSIQQAV-KOLCDFICSA-N | ||
| Standard InChI | InChI=1S/C12H18O2/c1-2-3-8-11-9-6-4-5-7-10(9)12(13)14-11/h7,9,11H,2-6,8H2,1H3/t9-,11+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Neocnidilide has insecticidal activity, it exhibits LC50 values of 9.90 micromol/mL of diet concentration against larvae of D. melanogaster. 2. Neocnidilide can enhance the skin penetration of benzoic acid. 3. Neocnidilide has antifungal activity. |
| Targets | Antifection |

Neocnidilide Dilution Calculator

Neocnidilide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1475 mL | 25.7374 mL | 51.4748 mL | 102.9495 mL | 128.6869 mL |
| 5 mM | 1.0295 mL | 5.1475 mL | 10.295 mL | 20.5899 mL | 25.7374 mL |
| 10 mM | 0.5147 mL | 2.5737 mL | 5.1475 mL | 10.295 mL | 12.8687 mL |
| 50 mM | 0.1029 mL | 0.5147 mL | 1.0295 mL | 2.059 mL | 2.5737 mL |
| 100 mM | 0.0515 mL | 0.2574 mL | 0.5147 mL | 1.0295 mL | 1.2869 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Glu(OBzl)-OBzl.HCl
Catalog No.:BCC2927
CAS No.:4561-10-8
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
- Isovouacapenol C
Catalog No.:BCN6557
CAS No.:455255-15-9
- 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone
Catalog No.:BCC8695
CAS No.:4551-69-3
- Grossamide K
Catalog No.:BCC4547
CAS No.:
- Corosolic acid
Catalog No.:BCN5503
CAS No.:4547-24-4
- Acetophenone tosylhydrazone
Catalog No.:BCC8804
CAS No.:4545-21-5
- kobe2602
Catalog No.:BCC5291
CAS No.:454453-49-7
- Nudifloside D
Catalog No.:BCN7005
CAS No.:454212-54-5
- PSB 11 hydrochloride
Catalog No.:BCC7239
CAS No.:453591-58-7
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- Saprirearine
Catalog No.:BCN3980
CAS No.:453518-30-4
- 3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride
Catalog No.:BCC8625
CAS No.:4568-71-2
- 5-O-Methylgenistein
Catalog No.:BCN7714
CAS No.:4569-98-6
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
- Pyridone 6
Catalog No.:BCC1874
CAS No.:457081-03-7
- PA 452
Catalog No.:BCC8005
CAS No.:457657-34-0
- BMS 470539 dihydrochloride
Catalog No.:BCC7850
CAS No.:457893-92-4
-
Scutebarbatine L
Catalog No.:BCN8369
CAS No.:960302-91-4
- Curcumin
Catalog No.:BCN5504
CAS No.:458-37-7
- Ilicic acid
Catalog No.:BCN5505
CAS No.:4586-68-9
- Boc-Asn-ONp
Catalog No.:BCC3072
CAS No.:4587-33-1
- SW033291
Catalog No.:BCC3981
CAS No.:459147-39-8
- JNJ-7777120
Catalog No.:BCC4543
CAS No.:459168-41-3
[Studies on the baths with crude drug: the effects of Senkyu extract as skin penetration enhancer].[Pubmed:1469611]
Yakugaku Zasshi. 1992 Sep;112(9):638-44.
The effects of Senkyu (Cnidii Rhizoma and Ligustici chuanxiong Rhizoma) on the drug skin penetration were studied to clarify its effectivity as the baths. Ether and methanol extracts, and some essential oils of Senkyu (i.e. ligustilide, Neocnidilide and butylidenephthalide) enhanced remarkably the skin penetration of benzoic acid. Furthermore, an appreciable correlation between the enhancing ratio and the skin/donor partition coefficient of benzoic acid was observed. These facts suggest that the constituents of Senkyu influence the skin penetration by enhancing the partition coefficient.
Larvicidal and adulticidal activity of alkylphthalide derivatives from rhizome of Cnidium officinale against Drosophila melanogaster.[Pubmed:15998112]
J Agric Food Chem. 2005 Jul 13;53(14):5549-53.
The insecticidal activity of the chloroform extract of Cnidium officinale rhizomes and its constituents was investigated against larvae and adults of Drosophila melanogaster and compared with that of rotenone. Bioassay-guided isolation of the chloroform extract of C. officinale resulted in the isolation and characterization of four alkylphthalides, cnidilide (1), (Z)-ligustilide (2), (3S)-butylphthalide (3), and Neocnidilide (4). The structures of these compounds were established by spectroscopic analysis. The isolated compounds 2, 3, and 4 exhibited LC50 values of 2.54, 4.99, and 9.90 micromol/mL of diet concentration against larvae of D. melanogaster, respectively. Against both sexes (males/females, 1:1) of adults (5-7 days old), compound 3 showed the most potent activity of the compounds isolated with the LD50 value of 5.93 microg/adult, comparable to that of rotenone (LD50 = 3.68 microg/adult). Structure-activity relationships of phthalides isolated suggest that the presence of conjugation with the carbonyl group in the lactone ring appeared to play an important role in the larvicidal activity. Acetylcholinesterase (prepared from the adult heads of D. melanogaster) inhibitory activity was also investigated in vitro to determine the insecticide mode of action for the acute adulticidal activity.
Isolation of the volatile fraction from Apium graveolens L. (Apiaceae) by supercritical carbon dioxide extraction and hydrodistillation: chemical composition and antifungal activity.[Pubmed:22974401]
Nat Prod Res. 2013;27(17):1521-7.
Apium graveolens L. (wild celery), belonging to the family of Apiaceae, is a scaposus hemicryptophyte. Instead, the cultivate plant is an annual or biennial herb widely used as a spice and seasoning in food. A broad range of biological activities have been attributed to A. graveolens. These include antimicrobial activity, larvicidal activity, hepatoprotective activity, nematicidal and mosquito repellent potential and antihyperlipidaemic properties.In this study, the authors compare the composition of the volatile fractions of A. graveolens collected in natural populations in Portugal and Italy and evaluate their potential as antifungal agents.The composition of the volatile oils obtained by hydrodistillation and their antifungal activity are reported. The oils were analysed by gas chromatography-flame ionisation detector and gas chromatography-mass spectrometry methods and their composition were compared with that of the volatile extracts isolated by supercritical CO2. A chemical variability in the extracts depending on the origin of the plants and on the extraction method was observed. The results showed the presence of sedanenolide, Neocnidilide and neophytadiene as main components. The minimal inhibitory concentration (MIC) and the minimal lethal concentration were used to evaluate the antifungal activity of the oils against Candida albicans, Candida tropicalis, Candida krusei, Candida guilliermondii, Candida parapsilosis, Cryptococcus neoformans, Trichophyton rubrum, Trichophyton mentagrophytes, T. mentagrophytes var. interdigitale, Trichophyton verrucosum, Microsporum canis, Microsporum gypseum, Epidermophyton floccosum, Aspergillus niger, Aspergillus fumigatus and Aspergillus flavus. The oil from Italy rich in neophytadiene is the more active, with MIC values of 0.04-0.64 microL mL(-1). Our results show that A. graveolens volatile extracts may be useful in the clinical treatment of fungal diseases.


