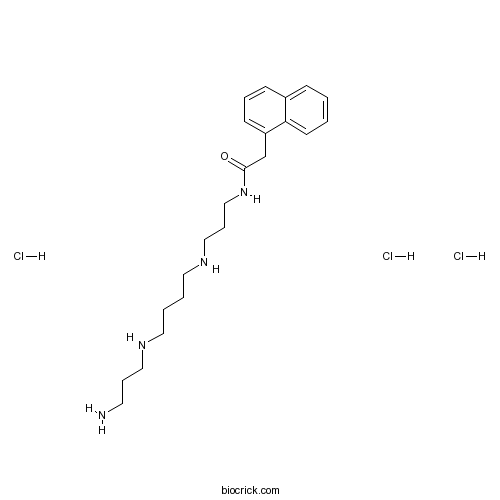
Naspm trihydrochlorideCAS# 1049731-36-3 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Vaniprevir
Catalog No.:BCC2030
CAS No.:923590-37-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1049731-36-3 | SDF | Download SDF |
| PubChem ID | 16219727 | Appearance | Powder |
| Formula | C22H37Cl3N4O | M.Wt | 479.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 1-Naphthyl acetyl spermine trihydrochloride | ||
| Solubility | H2O : 50 mg/mL (104.19 mM; Need ultrasonic) DMSO : 6.4 mg/mL (13.34 mM; Need ultrasonic) | ||
| Chemical Name | N-[3-[4-(3-aminopropylamino)butylamino]propyl]-2-naphthalen-1-ylacetamide;trihydrochloride | ||
| SMILES | C1=CC=C2C(=C1)C=CC=C2CC(=O)NCCCNCCCCNCCCN.Cl.Cl.Cl | ||
| Standard InChIKey | JNEOJAUJWOPWJS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H34N4O.3ClH/c23-12-6-15-24-13-3-4-14-25-16-7-17-26-22(27)18-20-10-5-9-19-8-1-2-11-21(19)20;;;/h1-2,5,8-11,24-25H,3-4,6-7,12-18,23H2,(H,26,27);3*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective antagonist of Ca2+-permeable AMPA receptors; blocks AMPA receptors lacking the GluA2 subunit. Protects hippocampal neurons against global ischemia-induced cell death. |

Naspm trihydrochloride Dilution Calculator

Naspm trihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0837 mL | 10.4186 mL | 20.8372 mL | 41.6745 mL | 52.0931 mL |
| 5 mM | 0.4167 mL | 2.0837 mL | 4.1674 mL | 8.3349 mL | 10.4186 mL |
| 10 mM | 0.2084 mL | 1.0419 mL | 2.0837 mL | 4.1674 mL | 5.2093 mL |
| 50 mM | 0.0417 mL | 0.2084 mL | 0.4167 mL | 0.8335 mL | 1.0419 mL |
| 100 mM | 0.0208 mL | 0.1042 mL | 0.2084 mL | 0.4167 mL | 0.5209 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Naspm (1-Naphthyl acetyl spermine) trihydrochloride, a synthetic analogue of Joro spider toxin, is a calcium permeable AMPA (CP-AMPA) receptors antagonist.
References:
[1]. Koike M, et al. Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res. 1997 Sep;29(1):27-36.
- 8-Hydroxydigitoxigenin
Catalog No.:BCN5864
CAS No.:1049674-06-7
- Ethyl β-D-ribo-hex-3-ulopyranoside
Catalog No.:BCC8977
CAS No.:104953-08-4
- H-D-Val-OtBu.HCl
Catalog No.:BCC3146
CAS No.:104944-18-5
- Tranilast Sodium
Catalog No.:BCC4091
CAS No.:104931-56-8
- Borapetoside B
Catalog No.:BCN6593
CAS No.:104901-05-5
- dl-Aloesol
Catalog No.:BCN7265
CAS No.:104871-04-7
- (+)-Isopulegol
Catalog No.:BCN4975
CAS No.:104870-56-6
- NF 023
Catalog No.:BCC6985
CAS No.:104869-31-0
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- A 331440 dihydrochloride
Catalog No.:BCC7963
CAS No.:1049740-32-0
- Cardiogenol C hydrochloride
Catalog No.:BCC7790
CAS No.:1049741-55-0
- PS 1145 dihydrochloride
Catalog No.:BCC7949
CAS No.:1049743-58-9
- 3-Acetoxy-4,7(11)-cadinadien-8-one
Catalog No.:BCN5865
CAS No.:104975-02-2
- Tacrolimus (FK506)
Catalog No.:BCC4952
CAS No.:104987-11-3
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Pallidol
Catalog No.:BCN3306
CAS No.:105037-88-5
- AST-1306 TsOH
Catalog No.:BCC4043
CAS No.:1050500-29-2
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
- GPR120 modulator 2
Catalog No.:BCC1600
CAS No.:1050506-87-0
- 1-Ketoaethiopinone
Catalog No.:BCN3219
CAS No.:105062-36-0
- Ro 51
Catalog No.:BCC6157
CAS No.:1050670-85-3
Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin's lymphoma.[Pubmed:17454628]
Leuk Lymphoma. 2007 Apr;48(4):708-15.
A phase I/II trial was performed to investigate the safety and tolerance of zosuquidar.3HCL, a potent inhibitor of P-glycoprotein (P-gp), when administered orally alone and in combination with the CHOP regimen in patients with untreated non-Hodgkin's lymphoma and to determine whether zosuquidar.3HCL affects pharmacokinetics of doxorubicin and vincristine. Doses of CHOP remained constant and the doses of zosuquidar.3HCL were increased from 200 to 500 mg per dose. A total of 15 patients were treated at three dose levels. A target dose providing peak and trough levels compatible with prolonged modulation of P-gp function was obtained in patients receiving three doses of 500 mg of zosuquidar.3HCL p.o. At this dose level, toxicity was minimal and no enhancement of CHOP-related toxicity was observed. Zosuquidar.3HCL did not significantly affect the pharmacokinetics of doxorubicin and had moderate effects on the pharmacokinetics of vincristine. Zosuquidar.3HCL can be safely administered with CHOP therapy using a 24-h schedule.
Clinical effects and P-glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine.[Pubmed:15257929]
Haematologica. 2004 Jul;89(7):782-90.
BACKGROUND AND OBJECTIVES: P-glycoprotein (P-gp) is a major cause of multidrug resistance (MDR) in acute myelogenous leukemia (AML) and is thought to contribute to the failure of chemotherapy. Zosuquidar trihydochloride (Z.3HCL) is a potent and selective inhibitor of P-gp which rapidly and effectively inhibits drug efflux. DESIGN AND METHODS: The aim of this study was to evaluate the clinical effects of Z.3HCL and determine its influence on P-gp activity. Sixteen AML patients were entered into a phase 1 dose ranging clinical trial of Z.3HCL, co-administered intravenously with daunorubicin and cytosine arabinoside (ARA-C). Clinical outcomes, toxicity abd adverse events were assessed. P-gp function was analyzed by flow cytometry. In vitro cytotoxicity was studied using the MTT assay. RESULTS: Eleven patients achieved a complete remission and one a partial remission with a median survival of 559 (range 38-906) days. Non-hematologic grade 3 and 4 toxicities were seen in 4 patients. Z.3HCL infusion was associated with rapid inhibition of Rh123 efflux in CD56+ cells in 16/16 patients and in CD33+ cells from 6/10 patients. The median inhibition was 95% for CD56+ cells and 85.25% for CD33+ cells was significantly elevated in 6/16 patients. The median IC50, using a MTT assay for daunorubicin, decreased significantly between Z.3HCL modulated and unmodulated cells (n=11,153 and 247 ng/mL respectively, p=0.01). INTERPRETATION AND CONCLUSIONS: The modulator Z.3HCL is a specific inhibitor of P-gp efflux and can be given safely to patients with AML in combination with induction doses of conventional cytotoxic drugs.
Stereochemistry of C-6 nucleophilic displacements on 1,1-difluorocyclopropyldibenzosuberanyl substrates. An improved synthesis of multidrug resistance modulator LY335979 trihydrochloride.[Pubmed:15497993]
J Org Chem. 2004 Oct 29;69(22):7653-60.
Studies of the displacement chemistry of 1,1-difluorocyclopropyldibenzosuberanyl alcohol 4 and its activated bromide derivative 6 have led to an improved approach to anti-2, a key precursor to LY335979 3HCl (1). Bromination of either syn-4 or anti-4 gave anti-oriented 6, indicating thermodynamically controlled product stereochemistry via a stabilized 1,1-difluorohomotropylium ion intermediate. Reaction of 6 with piperazine proceeded irreversibly to provide an isomeric mixture of piperazine products, with the syn:anti product ratio increased by solvent effects. Reaction of 6 with pyridine and pyrazine, on the other hand, gave anti-pyridinium and pyrazinium salts, respectively, apparently via equilibration of initially formed syn products. Reduction of pyrazinium salt 11 with lithium borohydride/TFA provided anti-2 unaccompanied by its syn isomer. A practical and expeditious approach to 1 was derived from these new results.
A Phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy.[Pubmed:15161679]
Clin Cancer Res. 2004 May 15;10(10):3265-72.
PURPOSE: Our intention was to (a) to investigate the safety and tolerability of a potent P-glycoprotein modulator, zosuquidar trihydrochloride (LY335979), when administered i.v. alone or in combination with doxorubicin, (b) to determine the pharmacokinetics of zosuquidar and correlate exposure to inhibition of P-glycoprotein function in a surrogate assay, and (c) to compare the pharmacokinetics of doxorubicin in the presence and absence of zosuquidar. PATIENTS AND METHODS: Patients with advanced malignancies who provided written informed consent received zosuquidar and doxorubicin administered separately during the first cycle of therapy and then concurrently in subsequent cycles. Zosuquidar was given i.v. over 48 h in a cohort-dose escalation manner until the occurrence of dose-limiting toxicity or protocol specified maximum exposure. Doxorubicin doses of 45, 60, 75 mg/m(2) were administered during the course of the trial. RESULTS: Dose escalation proceeded through 9 cohorts with a total of 40 patients. The maximal doses administered were 640 mg/m(2) of zosuquidar and 75 mg/m(2) of doxorubicin. No dose-limiting toxicity of zosuquidar was observed. Pharmacokinetic analysis revealed that, in the presence of zosuquidar at doses that exceeded 500 mg, there was a modest decrease in clearance (17-22%) and modest increase in area under the curve (15-25%) of doxorubicin. This change was associated with an enhanced leukopenia and thrombocytopenia but was without demonstrable clinical significance. The higher doses of zosuquidar were associated with maximal P-glycoprotein inhibition in natural killer cells. CONCLUSION: Zosuquidar can be safely coadministered with doxorubicin using a 48 h i.v. dosing schedule.
Blocking effect of 1-naphthyl acetyl spermine on Ca(2+)-permeable AMPA receptors in cultured rat hippocampal neurons.[Pubmed:9293490]
Neurosci Res. 1997 Sep;29(1):27-36.
Effects of 1-naphthyl acetyl spermine (NASPM), a synthetic analogue of Joro spider toxin (JSTX), on alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors were studied in cultured rat hippocampal neurons using the whole-cell patch clamp technique. A population of cultured neurons had AMPA receptors with a strong inward rectification and a high permeability to Ca2+ (type II neurons). Whereas most neurons (type I neurons) had AMPA receptors with a slight outward rectification and little Ca2+ permeability. NASPM selectively suppressed the inwardly rectifying and Ca(2+)-permeable AMPA receptors expressed in type II neurons. It had no effect on AMPA receptors in type I neurons. The blocking effect of NASPM on the Ca(2+)-permeable AMPA receptors was use and voltage-dependent. When the effect of NASPM reached a steady state, current responses induced by ionophoretic applications of kainate, a non-desensitizing agonist of AMPA receptors, in type II neurons were suppressed by NASPM in a dose-dependent manner at -60 mV (IC50 0.33 microM, and Hill coefficient 0.94). The response to kainate recovered partially after washing out NASPM. NASPM did not affect the Ca(2+)-permeable AMPA receptors when the neuronal membrane was held at potentials more positive than +40 mV. Furthermore, the blockade by NASPM which was attained at negative potentials was transiently removed by shifting membrane potential to +60 mV for 5 s together with a single ionophoretic application of kainate. NASPM would be useful as a pharmacological tool for elucidating both physiological and pathological significances of Ca(2+)-permeable AMPA receptors in the CNS.
Effects of a spider toxin and its analogue on glutamate-activated currents in the hippocampal CA1 neuron after ischemia.[Pubmed:7472325]
J Neurophysiol. 1995 Jul;74(1):218-25.
1. We studied the effects of polyamine toxins derived from a spider venom on CA1 pyramidal neurons in gerbil hippocampal slices by patch-clamp recording. Joro spider toxin (JSTX) and its synthetic analogue, 1-naphthyl acetyl spermine (Naspm), which are known to block non-N-methyl-D-aspartate (non-NMDA) receptor in a subunit specific manner, were used. 2. Naspm depressed the excitatory postsynaptic currents (EPSCs) mediated by non-NMDA receptor channels. A further reduction of EPSCs occurred with addition of 6-cyano-7-nitroquin-oxaline-2,3- dione (CNQX). Conversely, when CNQX was applied first, no further depression of EPSCs occurred on addition of Naspm, indicating that Naspm blocks a fraction of the CNQX-sensitive non-NMDA-receptor-mediated currents. 3. After ischemia, the time course of EPSCs of CA1 pyramidal neurons was slowed and Naspm depressed the slow EPSCs more strongly than those in control neurons. 4. Analysis of single-channel currents by outside-out patch-clamp recording from ischemic CA1 neurons revealed that Naspm blocked a subpopulation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate- and kainate-induced single-channel currents. 5. Because the EPSCs in CA1 neurons after ischemia are mediated by Ca(2+)-permeable non-NMDA receptor-mediated conductances, the present results indicate that Naspm and JSTX are effective at blocking abnormal EPSCs that may induce Ca2+ accumulation leading to delayed neuronal death after transient ischemic insult.


