NanchangmycinPolyether antibiotic CAS# 65101-87-3 |
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65101-87-3 | SDF | Download SDF |
| PubChem ID | 86278937 | Appearance | Powder |
| Formula | C48H80O13 | M.Wt | 865.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Nanchangmycin A | ||
| Solubility | DMSO : ≥ 30 mg/mL (33.74 mM) *"≥" means soluble, but saturation unknown. | ||
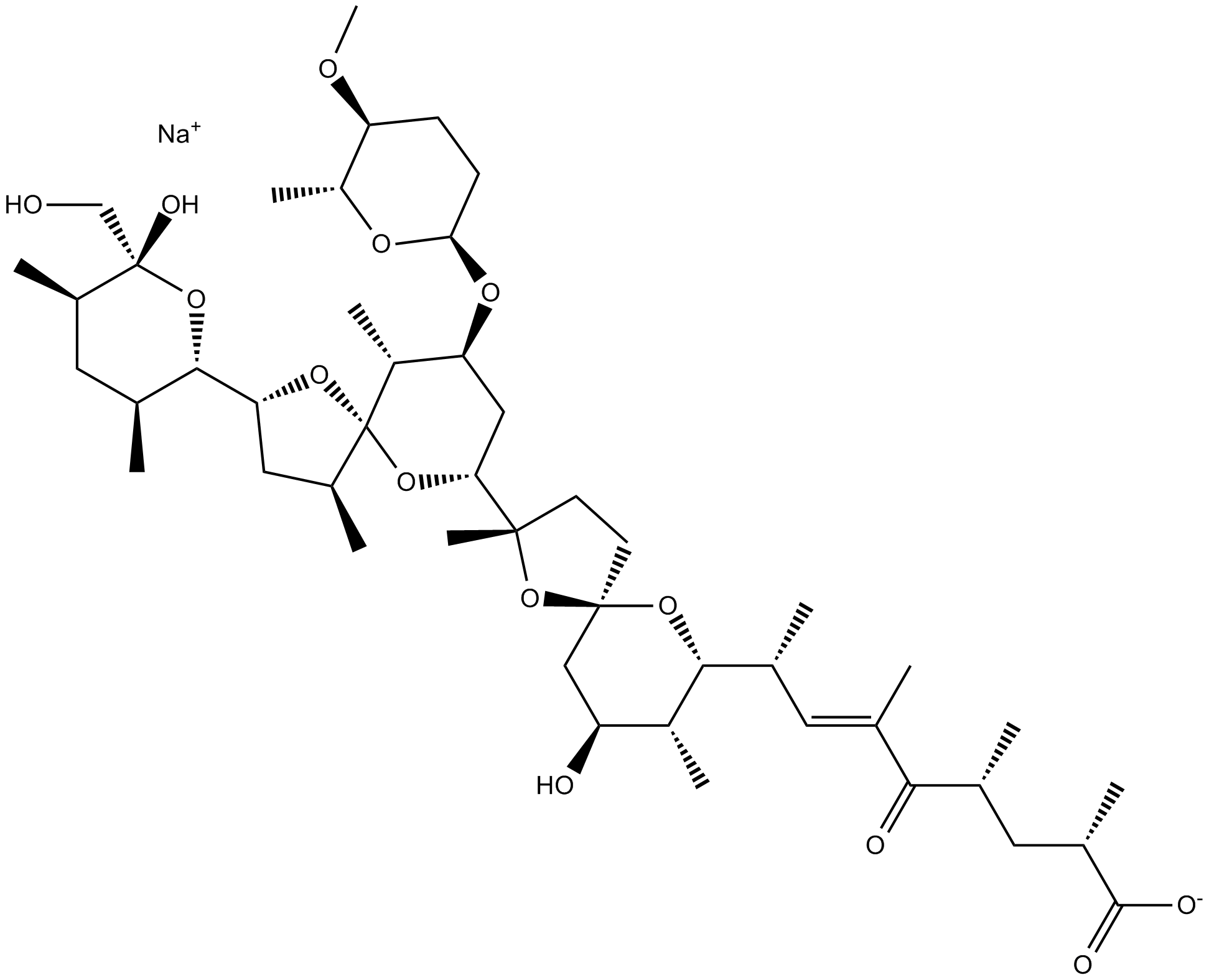
| Chemical Name | sodium;(E,2S,4R,8S)-8-[(2S,5R,7S,8R,9R)-7-hydroxy-2-[(2R,4S,5S,7R,9S,10R)-2-[(2S,3S,5R,6R)-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyloxan-2-yl]-9-[(2S,5S,6R)-5-methoxy-6-methyloxan-2-yl]oxy-4,10-dimethyl-1,6-dioxaspiro[4.5]decan-7-yl]-2,8-dimethyl-1,10-dioxaspiro[4.5]decan-9-yl]-2,4,6-trimethyl-5-oxonon-6-enoate | ||
| SMILES | CC1CC(C(OC1C2CC(C3(O2)C(C(CC(O3)C4(CCC5(O4)CC(C(C(O5)C(C)C=C(C)C(=O)C(C)CC(C)C(=O)[O-])C)O)C)OC6CCC(C(O6)C)OC)C)C)(CO)O)C.[Na+] | ||
| Standard InChIKey | XMAIRYYXDCNFKP-SEDNIUBGSA-M | ||
| Standard InChI | InChI=1S/C47H78O14.Na/c1-24(40(50)25(2)18-28(5)43(51)52)17-26(3)41-31(8)34(49)22-45(59-41)16-15-44(11,61-45)38-21-36(56-39-14-13-35(54-12)33(10)55-39)32(9)47(58-38)30(7)20-37(57-47)42-27(4)19-29(6)46(53,23-48)60-42;/h17,25-39,41-42,48-49,53H,13-16,18-23H2,1-12H3,(H,51,52);/q;+1/p-1/b24-17+;/t25-,26+,27+,28+,29-,30+,31-,32-,33-,34+,35+,36+,37-,38-,39-,41-,42+,44+,45-,46+,47+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antiviral. Inhibits Zika virus entry in a range of cell types including primary placental fibroblast cells, HUVECs and UtMECs (IC50 values are 0.1, 0.4 and 0.97 μM in U2OS, HBMECs and Jeg-3 cells respectively). Also inhibits cellular uptake of West Nile, dengue, sindbis and chikungunya viruses. |

Nanchangmycin Dilution Calculator

Nanchangmycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1559 mL | 5.7797 mL | 11.5594 mL | 23.1187 mL | 28.8984 mL |
| 5 mM | 0.2312 mL | 1.1559 mL | 2.3119 mL | 4.6237 mL | 5.7797 mL |
| 10 mM | 0.1156 mL | 0.578 mL | 1.1559 mL | 2.3119 mL | 2.8898 mL |
| 50 mM | 0.0231 mL | 0.1156 mL | 0.2312 mL | 0.4624 mL | 0.578 mL |
| 100 mM | 0.0116 mL | 0.0578 mL | 0.1156 mL | 0.2312 mL | 0.289 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nanchangmycin (dianemycin) is a polyether antibiotic with similar structure to dianemycin and is very active against a broad spectrum of harmful nematodes and insects but not for for mammals and plants.
- Sulfameter
Catalog No.:BCC4855
CAS No.:651-06-9
- L-152,804
Catalog No.:BCC7041
CAS No.:6508-43-6
- 6-Amino-2-methylquinoline
Catalog No.:BCC8759
CAS No.:65079-19-8
- Nimorazole
Catalog No.:BCC5253
CAS No.:6506-37-2
- Boc-His(Tos)-OH.DCHA
Catalog No.:BCC2605
CAS No.:65057-34-3
- VGX-1027
Catalog No.:BCC5203
CAS No.:6501-72-0
- 3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No.:BCN1387
CAS No.:65008-17-5
- 1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No.:BCN7347
CAS No.:65008-02-8
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- Benzoic acid
Catalog No.:BCN4201
CAS No.:65-85-0
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- NGR peptide
Catalog No.:BCC4418
CAS No.:651328-78-8
- N-Methylcorydaldine
Catalog No.:BCN3300
CAS No.:6514-05-2
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
- Elastase Inhibitor, SPCK
Catalog No.:BCC1226
CAS No.:65144-34-5
- 7-Hydroxyflavanone
Catalog No.:BCN6539
CAS No.:6515-36-2
- 4'-Hydroxyflavanone
Catalog No.:BCN6548
CAS No.:6515-37-3
- (16R)-Dihydrositsirikine
Catalog No.:BCN4195
CAS No.:6519-26-2
- (16R)-E-Isositsirikine
Catalog No.:BCN4000
CAS No.:6519-27-3
- Avermectin B1a
Catalog No.:BCC1382
CAS No.:65195-55-3
- Avermectin B1b
Catalog No.:BCC1383
CAS No.:65195-56-4
- Isosorbide
Catalog No.:BCC4667
CAS No.:652-67-5
- Gossypin
Catalog No.:BCN7987
CAS No.:652-78-8
Genetic engineering of Streptomyces bingchenggensis to produce milbemycins A3/A4 as main components and eliminate the biosynthesis of nanchangmycin.[Pubmed:24077727]
Appl Microbiol Biotechnol. 2013 Dec;97(23):10091-101.
Milbemycins A3/A4 are important 16-membered macrolides which have been commercialized and widely used as pesticide and veterinary medicine. However, similar to other milbemycin producers, the production of milbemycins A3/A4 in Streptomyces bingchenggensis is usually accompanied with undesired by-products such as C5-O - methylmilbemycins B2/B3 (alpha-class) and beta1/beta2 (beta-class) together with Nanchangmycin. In order to obtain high yield milbemycins A3/A4-producing strains that produce milbemycins A3/A4 as main components, milD, a putative C5-O-methyltransferase gene of S. bingchenggensis , was biofunctionally investigated by heterologous expression in Escherichia coli . Enzymatic analysis indicated that MilD can catalyze both alpha-class (A3/A4) and beta-class milbemycins (beta11) into C5-O-methylmilbemycins B2/B3 and beta1, respectively, suggesting little effect of furan ring formed between C6 and C8a on the C5-O-methylation catalyzed by MilD. Deletion of milD gene resulted in the elimination of C5-Omethylmilbemycins B2/B3 and beta1/beta2 together with an increased yield of milbemycins A3/A4 in disruption strain BCJ13. Further disruption of the gene nanLD encoding loading module of polyketide synthase responsible for the biosynthesis of Nanchangmycin led to strain BCJ36 that abolished the production of Nanchangmycin. Importantly, mutant strain BCJ36 (DeltamilDDeltananLD) produced milbemycins A3/A4 as main secondary metabolites with a yield of 2312 +/- 47 mug/ml, which was approximately 74 % higher than that of the initial strain S. bingchenggensis BC-109-6 (1326 +/- 37 mug/ml).
Screening Bioactives Reveals Nanchangmycin as a Broad Spectrum Antiviral Active against Zika Virus.[Pubmed:28099856]
Cell Rep. 2017 Jan 17;18(3):804-815.
Zika virus is an emerging arthropod-borne flavivirus for which there are no vaccines or specific therapeutics. We screened a library of 2,000 bioactive compounds for their ability to block Zika virus infection in three distinct cell types with two different strains of Zika virus. Using a microscopy-based assay, we validated 38 drugs that inhibited Zika virus infection, including FDA-approved nucleoside analogs. Cells expressing high levels of the attachment factor AXL can be protected from infection with receptor tyrosine kinase inhibitors, while placental-derived cells that lack AXL expression are insensitive to this inhibition. Importantly, we identified Nanchangmycin as a potent inhibitor of Zika virus entry across all cell types tested, including physiologically relevant primary cells. Nanchangmycin also was active against other medically relevant viruses, including West Nile, dengue, and chikungunya viruses that use a similar route of entry. This study provides a resource of small molecules to study Zika virus pathogenesis.
Essential role of the donor acyl carrier protein in stereoselective chain translocation to a fully reducing module of the nanchangmycin polyketide synthase.[Pubmed:22229794]
Biochemistry. 2012 Jan 31;51(4):879-87.
Incubation of recombinant module 2 of the polyether Nanchangmycin synthase (NANS), carrying an appended thioesterase domain, with the ACP-bound substrate (2RS)-2-methyl-3-ketobutyryl-NANS_ACP1 (2-ACP1) and methylmalonyl-CoA in the presence of NADPH gave diastereomerically pure (2S,4R)-2,4-dimethyl-5-ketohexanoic acid (4a). These results contrast with the previously reported weak discrimination by NANS module 2+TE between the enantiomers of the corresponding N-acetylcysteamine-conjugated substrate analogue (+/-)-2-methyl-3-ketobutyryl-SNAC (2-SNAC), which resulted in formation of a 5:3 mixture of 4a and its (2S,4S)-diastereomer 4b. Incubation of NANS module 2+TE with 2-ACP1 in the absence of NADPH gave unreduced 3,5,6-trimethyl-4-hydroxypyrone (3) with a k(cat) of 4.4 +/- 0.9 min(-)(1) and a k(cat)/K(m) of 67 min(-)(1) mM(-)(1), corresponding to a approximately 2300-fold increase compared to the k(cat)/K(m) for the diffusive substrate 2-SNAC. Covalent tethering of the 2-methyl-3-ketobutyryl thioester substrate to the NANS ACP1 domain derived from the natural upstream PKS module of the Nanchangmycin synthase significantly enhanced both the stereospecificity and the kinetic efficiency of the sequential polyketide chain translocation and condensation reactions catalyzed by the ketosynthase domain of NANS module 2.
The biosynthesis of the polyether antibiotic nanchangmycin is controlled by two pathway-specific transcriptional activators.[Pubmed:22109812]
Arch Microbiol. 2012 Jun;194(6):415-26.
The Nanchangmycin (NAN) produced by Streptomyces nanchangensis NS3226 is a polyether antibiotic resembling monensin in their gene clusters and the chemical structures. They can inhibit gram-positive bacteria and be a growth promoter for ruminants. Within the Nanchangmycin gene cluster (nan), we identified that two SARP-family regulatory genes, nanR1 and nanR2, were both required to activate the transcription of all nan polyketide genes. Overexpression of NanR1 and NanR2 in wild-type increase NAN yields by at least three folds. Bioinformatic analysis of the immediate upstream DNA sequence of each nan gene and quantitative real-time RT-PCR analysis of the nan operons identified five putative SARP binding sites. Moreover, deletion of an AraC-family repressor gene nanR4 increased expression of NanR1 and R2 and led to a threefold increase in NAN production.


