NPPBinhibitor of chloride channel CAS# 107254-86-4 |
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107254-86-4 | SDF | Download SDF |
| PubChem ID | 4549 | Appearance | Powder |
| Formula | C16H16N2O4 | M.Wt | 300.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (499.48 mM; Need ultrasonic and warming) | ||
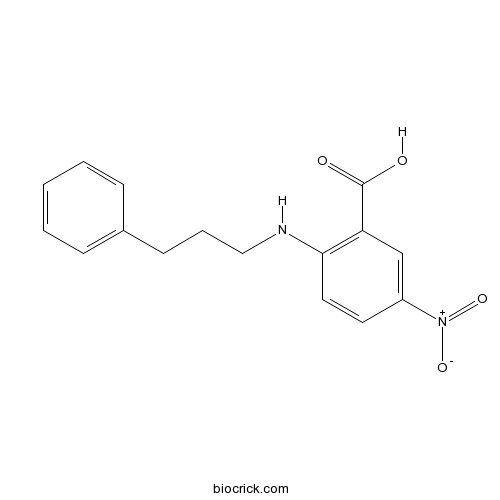
| Chemical Name | 5-nitro-2-(3-phenylpropylamino)benzoic acid | ||
| SMILES | C1=CC=C(C=C1)CCCNC2=C(C=C(C=C2)[N+](=O)[O-])C(=O)O | ||
| Standard InChIKey | WBSMIPAMAXNXFS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16N2O4/c19-16(20)14-11-13(18(21)22)8-9-15(14)17-10-4-7-12-5-2-1-3-6-12/h1-3,5-6,8-9,11,17H,4,7,10H2,(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibits calcium-sensitive chloride currents (10 μM). Putative GPR35 agonist. |

NPPB Dilution Calculator

NPPB Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3299 mL | 16.6495 mL | 33.2989 mL | 66.5978 mL | 83.2473 mL |
| 5 mM | 0.666 mL | 3.3299 mL | 6.6598 mL | 13.3196 mL | 16.6495 mL |
| 10 mM | 0.333 mL | 1.6649 mL | 3.3299 mL | 6.6598 mL | 8.3247 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.6649 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NPPB, 5-nitro-2-(3-phenylpropylamino)-benzoic acid, is a potent inhibitor of chloride channel with IC50 of 80 nM for the short circuit current.[1]
Chloride channel blockers possess several sites of interaction, including the negatively charged carboxylate group, the secondary amine group which probably carries a positive partial charge, and for the very potent agents like NPPB an additional negative partial charge at the -NO2 substituent. In addition, an apolar interaction with a cycloaryl residue is necessary, and this site of interaction has a specific spacing from the secondary amino nitrogen.[1]
NPPB was evaluated for the activity on the equivalent short circuit current, corresponding to the secondary active transport of Cl- and measurements of the voltage across the basolateral membrane. The result revealed that NPPB possessed a good potency with IC50 of 80 nM for inhibiting the short circuit current. Furthermore, NPPB was also tested for its activity on various anion channels. Adopting freshly-isolated cells from the rat portal vein, the effects of NPPB were investigated on evoked and spontaneous currents by use of conventional whole-cell recording and perforated-patch techniques. At a holding potential of -60 mV in potassium-free, caesium-containing solutions, NPPB (10 μM) inhibited Ca-sensitive chloride currents (ICI(Ca)) evoked by caffeine (10 mM) and by noradrenaline (10μM) by the extend of 58% and 96%, respectively. In addition, at a holding potential of -2 mV in potassium -containing solutions, NPPB (10 μM) inhibited charybdotoxin-sensitive potassium-currents (IBK(Ca)) induced by noradrenaline (10 μM) and acetylcholine (10 μM) by approximately 90%. NPPB’s inhibitory effects of volume-activated taurine, glucose, and uridine influxes was studied. The IC50 for the inhibition of the volume- activated fluxes by NPPB was around 12 μM. [1-3]
References:
[1] Wangemann, Ph, et al. "Cl−-channel blockers in the thick ascending limb of the loop of Henle Structure activity relationship." Pflügers Archiv 407.2 (1986): S128-S141.
[2] Kirkup, A. J., G. Edwards, and A. H. Weston. "Investigation of the effects of 5‐nitro‐2‐(3‐phenylpropylamino)‐benzoic acid (NPPB) on membrane currents in rat portal vein." British journal of pharmacology 117.1 (1996): 175-183.
[3] Kirk, Kiaran, J. C. Ellory, and J. D. Young. "Transport of organic substrates via a volume-activated channel." Journal of Biological Chemistry 267.33 (1992): 23475-23478.
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- 8,9-Dihydroxy-10-isobutyryloxythymol
Catalog No.:BCN7974
CAS No.:107109-97-7
- Adipic dihydrazide
Catalog No.:BCC8810
CAS No.:1071-93-8
- Apo-12'-Lycopenal
Catalog No.:BCC8298
CAS No.:1071-52-9
- EIT hydrobromide
Catalog No.:BCC6824
CAS No.:1071-37-0
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- Defactinib
Catalog No.:BCC5494
CAS No.:1073154-85-4
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
Oncogenic targets Mmp7, S100a9, Nppb and Aldh1a3 from transcriptome profiling of FAP and Pirc adenomas are downregulated in response to tumor suppression by Clotam.[Pubmed:27706811]
Int J Cancer. 2017 Jan 15;140(2):460-468.
Intervention strategies in familial adenomatous polyposis (FAP) patients and other high-risk colorectal cancer (CRC) populations have highlighted a critical need for endoscopy combined with safe and effective preventive agents. We performed transcriptome profiling of colorectal adenomas from FAP patients and the polyposis in rat colon (Pirc) preclinical model, and prioritized molecular targets for prevention studies in vivo. At clinically relevant doses in the Pirc model, the drug Clotam (tolfenamic acid, TA) was highly effective at suppressing tumorigenesis both in the colon and in the small intestine, when administered alone or in combination with Sulindac. Cell proliferation in the colonic crypts was reduced significantly by TA, coincident with increased cleaved caspase-3 and decreased Survivin, beta-catenin, cyclin D1 and matrix metalloproteinase 7. From the list of differentially expressed genes prioritized by transcriptome profiling, Mmp7, S100a9, NPPB and Aldh1a3 were defined as key oncogene candidates downregulated in colon tumors after TA treatment. Monthly colonoscopies revealed the rapid onset of tumor suppression by TA in the Pirc model, and the temporal changes in Mmp7, S100a9, NPPB and Aldh1a3, highlighting their value as potential early biomarkers for prevention in the clinical setting. We conclude that TA, an "old drug" repurposed from migraine, offers an exciting new therapeutic avenue in FAP and other high-risk CRC patient populations.
Correlation between rs198388 and rs198389 polymorphismsin brainnatriuretic peptide (NPPB) gene and susceptibility to congenital heart diseases in a Chinese population.[Pubmed:26770549]
Int J Clin Exp Med. 2015 Oct 15;8(10):19162-6. eCollection 2015.
OBJECTIVE: We discussed the correlation between SNP loci (rs198389 and rs198388) in brain natriuretic peptide gene (NPPB) and susceptibility to congenital heart diseases (CHD). METHOD: Multiplex SNaPshot technique was adopted for profiling of SNP genotypes at loci rs198389 and rs198388 in NPPB gene among 150 cases of CHDand 150 normal controls. RESULTS: The distribution frequency of 3 genotypes (AA, AG and GG) at locus rs198389 was 40.7%, 36.0% and 23.3% in CHD group, respectively, showing significant differences compared with the normal controls (P<0.001). Gallele was associated with higher risk of CHD (OR=2.48, 95% CI=1.77-3.48). The distribution frequency of CC, CTand TT genotypes at locus rs198388 was 60.7%, 17.3% and 22.0% in CHD group, respectively, also showing significant differences compared with the normal controls (P<0.001). C allele could increase the risk of CHD (OR=1.92, 95% CI=1.48-2.48). CONCLUSION: SNP loci rs198389 and rs198388 in NPPB gene were correlated with genetic susceptibility to CHD.
Identification of a regulatory domain controlling the Nppa-Nppb gene cluster during heart development and stress.[Pubmed:27048739]
Development. 2016 Jun 15;143(12):2135-46.
The paralogous genes Nppa and NPPB are organized in an evolutionarily conserved cluster and provide a valuable model for studying co-regulation and regulatory landscape organization during heart development and disease. Here, we analyzed the chromatin conformation, epigenetic status and enhancer potential of sequences of the Nppa-NPPB cluster in vivo Our data indicate that the regulatory landscape of the cluster is present within a 60-kb domain centered around NPPB Both promoters and several potential regulatory elements interact with each other in a similar manner in different tissues and developmental stages. The distribution of H3K27ac and the association of Pol2 across the locus changed during cardiac hypertrophy, revealing their potential involvement in stress-mediated gene regulation. Functional analysis of double-reporter transgenic mice revealed that Nppa and NPPB share developmental, but not stress-response, enhancers, responsible for their co-regulation. Moreover, the NPPB promoter was required, but not sufficient, for hypertrophy-induced Nppa expression. In summary, the developmental regulation and stress response of the Nppa-NPPB cluster involve the concerted action of multiple enhancers and epigenetic changes distributed across a structurally rigid regulatory domain.
An NPPB Promoter Polymorphism Associated With Elevated N-Terminal pro-B-Type Natriuretic Peptide and Lower Blood Pressure, Hypertension, and Mortality.[Pubmed:28341776]
J Am Heart Assoc. 2017 Mar 24;6(4). pii: JAHA.116.005257.
BACKGROUND: Elevated B-type natriuretic peptide (BNP) levels are associated with heart failure and increased mortality in the general population. We investigated rs198389, a functional variant in the promoter region of the BNP gene (NPPB), in patients from the Atherosclerosis Risk in Communities Study to investigate associations with N-terminal pro-BNP (NT-proBNP) levels and outcomes. METHODS AND RESULTS: A total of 11 361 black and white patients with rs198389 genotyping attended visit 1 (aged 45-64 years; 1987-1989), with follow-up visits occurring every 3 years (visit 2-visit 4, 1990-1999), followed by visit 5 (2011-2013). NT-proBNP levels were measured at visits 2, 4, and 5. At visit 2, the GG genotype (frequency 18%) was associated with a 41% higher mean plasma level of NT-proBNP compared with the AA genotype (frequency 34%), with intermediate values observed in AGs (P=4.2x10(-52)). The GG genotype was associated with reduced systolic blood pressure (-1.6 mm Hg, P=0.006), diastolic blood pressure (-1 mm Hg, P=0.003), antihypertension medication use (odds ratio, 0.85; 95% CI, 0.74-0.97 [P=0.02]), and hypertension (odds ratio, 0.81; 95% CI, 0.72-0.92 [P=0.002]) compared with the AA genotype with intermediate values in AGs. These relationships persisted throughout subsequent visits. After a median follow-up of 23 years, there were 4031 deaths. With and without covariate adjustment, the GG genotype was associated with modestly lower mortality (hazard ratio, 0.86; 95% CI, 0.78-0.95), primarily reflective of cardiovascular death (hazard ratio, 0.75; 95% CI, 0.61-0.92), and increased residual lifespan of 8 months from 50 years of age (P=0.02) versus AAs. CONCLUSIONS: The rs198389 G allele in the NPPB promoter is associated with elevated levels of NT-proBNP throughout adult life, reduced blood pressure, hypertension and cardiovascular mortality, and increased lifespan.
5-Nitro-2-(3-phenylpropylamino)benzoic acid is a GPR35 agonist.[Pubmed:18818509]
Pharmacology. 2008;82(4):245-9.
GPR35 is a Gi/o- and G16-coupled receptor abundantly expressed in gastrointestinal tissues and immune cells. Kynurenic acid (a tryptophan metabolite and ionotropic glutamate receptor antagonist) and zaprinast (a phosphodiesterase inhibitor) are GPR35 agonists. Here, we show that the chloride channel blocker 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) is also a GPR35 agonist. NPPB activates the GPR35-Gi/o and GPR35-G16 pathways in human embryonic kidney 293 (HEK293) cells and induces intracellular calcium mobilization in a concentration-dependent manner in HEK293 cells coexpressing human, rat or mouse GPR35 and the chimeric G protein G(qi5). These results suggest a novel pharmacological activity of NPPB and will provide useful information to search for more potent and selective GPR35 agonists.
Investigation of the effects of 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) on membrane currents in rat portal vein.[Pubmed:8825360]
Br J Pharmacol. 1996 Jan;117(1):175-83.
1. The effects of 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) were investigated on evoked and spontaneous currents in freshly-isolated cells from the rat portal vein by use of conventional whole-cell recording and perforated-patch techniques. 2. At a holding potential of -60 mV in potassium-free, caesium-containing solutions, NPPB (10 microM) inhibited calcium (Ca)-sensitive chloride currents (ICl(Ca)) evoked by caffeine (10 mM) and by noradrenaline (10 microM) by 58% and 96%, respectively. 3. At a holding potential of -2 mV in potassium (K)-containing solutions, NPPB (10 microM) inhibited charybdotoxin-sensitive K-currents (IBK(Ca)) induced by noradrenaline (10 microM) and acetylcholine (10 microM) by approximately 90%. In contrast, IBK(Ca) induced by caffeine (10 mM) was unaffected in the presence of NPPB (10 microM). Conversely, IBK(Ca) elicited by caffeine (2 mM) was reduced by approximately 50% whereas IBK(Ca) evoked by noradrenaline (50 microM) was not significantly inhibited by NPPB. 4. In K-containing solutions, NPPB (10 microM) abolished spontaneous transient outward currents (STOCs) and induced a slowly-developing outward K-current. Bath application of glibenclamide (10 microM) abolished the outward current but did not antagonize the inhibitory effects of NPPB on STOCs or on IBK(Ca) evoked by noradrenaline. 5. In caesium-containing solutions, NPPB (30 microM) inhibited voltage-sensitive Ca-currents. 6. In Ca-free, K-containing solutions and in the presence of glibenclamide (5 microM), IBK(Ca) induced by 20 microM NS1619 was enhanced by NPPB (10 microM). 7. It is concluded that NPPB inhibits agonist-induced ICl(Ca) in rat portal vein smooth muscle. However, this agent also inhibits agonist-evoked IBK(Ca) and STOCs. Moreover, NPPB inhibits voltage-sensitive Ca-currents and stimulates a glibenclamide-sensitive K-current and IBK(Ca). The effects of this agent on evoked ICl(Ca) and IBK(Ca) and on STOCs probably involves an inhibitory action on intracellular Ca-stores.
Effects of NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid) on chloride transport in intestinal tissues and the T84 cell line.[Pubmed:1720331]
Biochim Biophys Acta. 1991 Nov 14;1115(1):42-8.
NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid) has been reported to block Cl- channels in isolated rabbit nephrons with high potency (IC50 = 80 nM). The effects of this compound on Cl(-)-mediated transport processes in intestinal tissues have been studied using agonist-stimulated short-circuit current (T84) in Ussing chamber experiments and 36Cl- fluxes in monolayers of a colonic cell line (T84). NPPB inhibited PGE1-stimulated Isc in rabbit distal colon and ileum at concentrations in the range 20 to 100 microM. However, NPPB at the same concentrations also inhibited glucose-stimulated Isc in rabbit ileum, suggesting that its effects were not restricted to those on Cl- transport. Consistent with this, exposure of rabbit distal colon to 100 microM NPPB was found to reduce endogenous ATP levels by 69%, implying that, at these concentrations, NPPB could impair active transport processes by an effect on cellular energy metabolism. Clear evidence for a direct effect of NPPB on epithelial chloride channels was found in studies on Cl- fluxes in T84 cell monolayers. NPPB inhibited VIP-stimulated Cl- uptake into T84 cells with an IC50 of 414 microM. NPPB (1 mM) also inhibited Cl- efflux from pre-loaded cells confirming its effect as a weak Cl- channel blocker in this system.
Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship.[Pubmed:2434915]
Pflugers Arch. 1986;407 Suppl 2:S128-41.
On the basis of our findings with diphenylamine-2-carboxylate we have searched for compounds which possess an even higher affinity for the Cl(-)-channels in the basolateral membrane of the thick ascending limb of the loop of Henle. To quantity the inhibitory potency, we performed measurements of the equivalent short circuit current, corresponding to the secondary active transport of Cl- and measurements of the voltage across the basolateral membrane. A survey of 219 compounds reveals that relatively simple modifications in the structure of diphenylamine-2-carboxylate led to very potent blockers such as 5-nitro-2-(3-phenylpropylamino)-benzoate which inhibits the short circuit current half maximally (IC50) at 8 X 10(-8) mol/l. A comparison of the structural formula and the respective IC50 values leads to several empirical conclusions: The potent compounds are lipophilic due to the apolar residue (e.g. phenyl- or cycloalkyl group). Replacing this part of the molecule by an aliphatic chain (up to 4 C-atoms) leads to inactive compounds. Most of the inhibitors are secondary amines. Linking other than with -NH- between the phenyl ring and the benzoic acid results in inactive compounds. Tertiary amines, such as in case of 2-(N,N-diphenylamine)benzoic acid or N-methylphenylamine-benzoic acid are poorly active. The carboxylate group of the benzoate moiety must be in ortho position to the amino group. Introduction of substituents into the benzoate moiety e.g. -NO2 (in meta position to the carboxylate group), or by -Cl (in para position to the carboxylate group) results in an increase of inhibitory potency. A -CH2-, -C2H4-, -C3H6- spacer between the amino bridge and the phenyl ring increases the affinity for the Cl(-)-channel by several orders of magnitude. The above described structure activity relationship renders it likely that these chloride channel blockers possess several sites of interaction: The negatively charged carboxylate group, the secondary amine group which probably carries a positive partial charge, and for the very potent agents (nos. 130, 143, 144, and 145) an additional negative partial charge at the respective -Cl or -NO2 substituent. Finally, also an apolar interaction with an cycloalkyl or cycloaryl residue seems to be required, and this site of interaction has a defined spacing from the secondary amino nitrogen.


