Musk ketoneCAS# 81-14-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81-14-1 | SDF | Download SDF |
| PubChem ID | 6669 | Appearance | White powder |
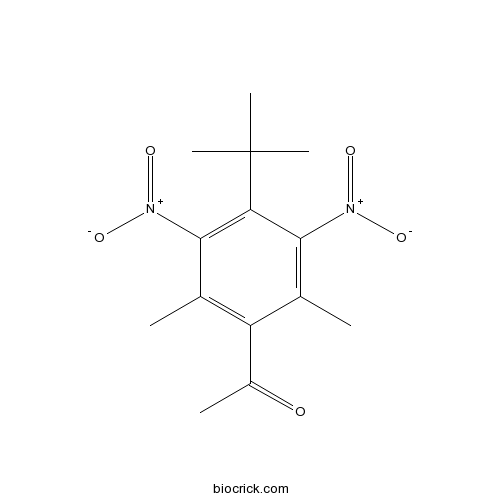
| Formula | C14H18N2O5 | M.Wt | 294.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(4-tert-butyl-2,6-dimethyl-3,5-dinitrophenyl)ethanone | ||
| SMILES | CC1=C(C(=C(C(=C1[N+](=O)[O-])C(C)(C)C)[N+](=O)[O-])C)C(=O)C | ||
| Standard InChIKey | WXCMHFPAUCOJIG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H18N2O5/c1-7-10(9(3)17)8(2)13(16(20)21)11(14(4,5)6)12(7)15(18)19/h1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Musk ketone Dilution Calculator

Musk ketone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3979 mL | 16.9895 mL | 33.9789 mL | 67.9579 mL | 84.9473 mL |
| 5 mM | 0.6796 mL | 3.3979 mL | 6.7958 mL | 13.5916 mL | 16.9895 mL |
| 10 mM | 0.3398 mL | 1.6989 mL | 3.3979 mL | 6.7958 mL | 8.4947 mL |
| 50 mM | 0.068 mL | 0.3398 mL | 0.6796 mL | 1.3592 mL | 1.6989 mL |
| 100 mM | 0.034 mL | 0.1699 mL | 0.3398 mL | 0.6796 mL | 0.8495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Amino-1-naphthalenesulfonic acid
Catalog No.:BCC8758
CAS No.:81-05-0
- 20R-Ginsenoside Rg2
Catalog No.:BCN2554
CAS No.:80952-72-3
- (20R)-Ginsenoside Rh1
Catalog No.:BCN3700
CAS No.:80952-71-2
- Zingibroside R1
Catalog No.:BCN3433
CAS No.:80930-74-1
- Gnetumontanin B
Catalog No.:BCN3756
CAS No.:809237-87-4
- Concanamycin A
Catalog No.:BCC3919
CAS No.:80890-47-7
- 3,22-Dihydroxyolean-12-en-29-oic acid
Catalog No.:BCN1347
CAS No.:808769-54-2
- Pyracrenic acid
Catalog No.:BCN7455
CAS No.:80832-44-6
- FG-4592 (ASP1517)
Catalog No.:BCC2227
CAS No.:808118-40-3
- 1-Hydroxycanthin-6-one
Catalog No.:BCN4347
CAS No.:80787-59-3
- 3-Acetylyunaconitine
Catalog No.:BCN7640
CAS No.:80787-51-5
- Tectorigenin sodium sulfonate
Catalog No.:BCN8161
CAS No.:807636-25-5
- 2-Amino-1-naphthalenesulfonic acid
Catalog No.:BCC8519
CAS No.:81-16-3
- Taurocholic acid
Catalog No.:BCN6954
CAS No.:81-24-3
- Cholic acid
Catalog No.:BCN1286
CAS No.:81-25-4
- Sennoside A
Catalog No.:BCN1002
CAS No.:81-27-6
- Purpurin
Catalog No.:BCN3477
CAS No.:81-54-9
- Warfarin
Catalog No.:BCC5221
CAS No.:81-81-2
- Rhodamine B
Catalog No.:BCN7215
CAS No.:81-88-9
- 4-Hydroxy-4-(methoxycarbonylmethyl)cyclohexanone
Catalog No.:BCN1346
CAS No.:81053-14-7
- Methyl 4-prenyloxycinnamate
Catalog No.:BCN7520
CAS No.:81053-49-8
- EUK 134
Catalog No.:BCC4317
CAS No.:81065-76-1
- Kauniolide
Catalog No.:BCC5313
CAS No.:81066-45-7
- Pravastatin
Catalog No.:BCC4141
CAS No.:81093-37-0
Assessing seasonal variation of synthetic musks in beach sands from Oporto coastal area: A case study.[Pubmed:28431318]
Environ Pollut. 2017 Jul;226:190-197.
Synthetic musk compounds are widely used in the formulation of several cosmetics, personal care and household products. Due to their massive and widespread use, together with some health concerns, they are considered emerging pollutants and have been detected in different environmental compartments. This study focused on the evaluation of the concentration of synthetic musks (five nitro, five polycyclic and one macrocyclic musks) in beach sands, from Oporto coastal area (Portugal), contributing to the enhancement of the knowledge of levels, trends and behaviour of these compounds in this particular matrix. To accomplish this task, a QuEChERS methodology ("Quick, Easy, Cheap, Effective, Rugged, and Safe") coupled to gas chromatography-mass spectrometry (GC-MS) was successfully used to determine synthetic musks from beach sand. The chosen methodology proved to be suitable, achieving satisfactory results for precision (relative standard deviation values below 15%), accuracy (average recovery of 97%) and limits of detection (below 38 pg g(-1)). Synthetic musks were detected in all 45 analysed samples, in concentrations ranging from 0.01 to 27 ng g(-1)dw. Tonalide (93%), exaltolide (89%) and galaxolide (76%) were the most commonly detected compounds, but also those detected in higher concentrations (up to 27 ng g(-1)dw). Musk ambrette, moskene, tibetene and xylene were not detected in any of the samples. Higher concentrations were as expected detected in the Summer (total average concentration of 9.21 ng g(-1)dw), namely in samples from Valadares Sul (29 ng g(-1)dw), Francelos (25 ng g(-1)dw) and Castelo do Queijo (25 ng g(-1)dw). The preliminary environmental risk assessment study based on the determination of hazard quotients revealed that the presence of analysed compounds (tonalide, galaxolide and Musk ketone) seems to pose no risk to the studied environmental compartment.
In vitro determination of transdermal permeation of synthetic musks and estimated dermal uptake through usage of personal care products.[Pubmed:28129620]
Chemosphere. 2017 Apr;173:417-424.
Synthetic musks, chemical constituents of personal care products, enter the human body through dermal contact. Elucidation of the mechanisms underlying transdermal permeation of synthetic musks should enhance our understanding of their uptake and distribution in human skin and allow accurate evaluation of associated human exposure. Here, the transdermal permeation dynamics and distribution of galaxolide (HHCB) and tonalide (AHTN) were investigated using an in vitro skin diffusion model. The transdermal permeation amounts of HHCB and AHTN increased rapidly during the first 6 h. The applied HHCB and AHTN amounts did not affect percutaneous absorption rates. HHCB and AHTN remained primarily in the stratum corneum, accounting for 70.0% and 70.3% of the totals during the 24-h period, respectively. The percutaneous absorption rate of both chemicals was approximately 11%. HHCB, AHTN, Musk ketone, musk xylene, and Musk-T were detected in 29 personal care products. The average total concentrations of the musks were 3990, 54.0, 17.7, and 9.8 mug g(-1) in perfume, shampoo, lotion, and shower gel, respectively. Among the four product categories, HHCB was dominant (57.4%-99.6%), followed by AHTN. The data clearly indicate that polycyclic and nitro musks are most commonly used in personal care products. The total estimated dermal intake (51.6 mug kg(-1)bw day(-1)) was markedly higher than total dermal uptake (5.9 mug kg(-1)bw day(-1)) when percutaneous absorption rates of the chemicals were added into the calculation. Uptake of HHCB and AHTN via dermal contact of personal care products was significantly higher than that from dust inhalation calculated according to earlier literature data.
Native musk and synthetic musk ketone strongly induced the growth repression and the apoptosis of cancer cells.[Pubmed:27931220]
BMC Complement Altern Med. 2016 Dec 8;16(1):511.
BACKGROUND: Musk is widely used in clinical practice for its anti-cancer properties. Here, we treated various types of cancer using musk to determine which cancers are sensitive to musk treatment. We also compared effects of native musk and synthetic Musk ketone in cancer cells. Furthermore, we investigated mechanisms underlying effects of musk. METHODS: Twenty two cancer cell lines were treated with musk. Cell proliferation and apoptosis analyses were carried out. Native musk and synthetic Musk ketone were analyzed by gas chromatograph-mass spectrometer (GC-MS) assay. Differentially expressed genes were determined by microarray and quantitative real-time polymerase chain reaction. RESULTS: Native musk strongly induced the growth repression and the apoptosis in the majority of cancer cell lines in a dose-dependent manner, but distinct types of cancer showed significantly different reactions. Cancer cells which originated from epithelial cells showed higher sensitivity for musk treatment. By contrast, leukaemia and lymphoma cells were not sensitive. GC-MS analysis demonstrated that native musk contains more than 30 contents in which Musk ketone is a major component; synthetic Musk ketone was consistent with natural Musk ketone, and the used sample of synthetic Musk ketone contained only sole component. Similar to native musk, synthetic Musk ketone induced the growth repression and the apoptosis of cancer cells. Additionally, numerous genes were differentially expressed in lung cancer cells after native musk treatment. These differentially expressed genes were involved in many signalling pathways. Among these pathways, apoptosis-related pathways included interleukin family, tumor necrosis factor family, and MAPK signalling pathway. Native musk and synthetic Musk ketone can up-regulate IL-24 (interleukin family) and DDIT3 (MAPK signalling pathway) in lung cancer cells. CONCLUSIONS: This research provided strong evidence that native musk and synthetic Musk ketone can induce the growth repression and the apoptosis of cancer cells. However, the selection of sensitive cancer patient for individualized treatment is a key step in clinical application. Synthetic Musk ketone can substitute for native musk to treat cancer patients. Musk might induce the growth repression and the apoptosis of lung cancer cells through up-regulating IL-24 and DDIT3 expressions.
Determination of 24 personal care products in fish bile using hybrid solvent precipitation and dispersive solid phase extraction cleanup with ultrahigh performance liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometry.[Pubmed:29650477]
J Chromatogr A. 2018 May 25;1551:29-40.
Personal care products (PCPs) are ubiquitous in aquatic environments owing to the continuous discharge of domestic wastewater from highly urbanized regions. These PCPs can be adsorbed by fish and thereafter usually enter the bile of the fish through biliary excretion. In this study, a sensitive method based on a combination of hybrid solvent precipitation and dispersive solid phase extraction (d-SPE) purification was developed to simultaneously extract and detect 24 PCPs, namely, 16 biocides, 4 synthetic musks, and 4 benzotriazoles, from fish bile. Hybrid precipitation on solid phase extraction (SPE) tubes was applied to remove phospholipids and proteins, and a d-SPE procedure was used for further purification. The extraction solvents for the hybrid precipitation/SPE tubes and d-SPE materials were optimized. The method performance for bile samples both with and without enzyme hydrolysis using beta-glucuronidase/aryl-sulfatase were validated. The 24 PCPs in fish bile were spiked with standard concentrations of 10ng/mL, 20ng/mL, 100ng/mL, and 200ng/mL to evaluate recoveries, which ranged from 70 to 120% for 16, 16, 22, and 21 analytes with hydrolysis, respectively, and 70-120% for 14, 15, 23, and 23 analytes without hydrolysis, respectively. The quantification limits for target PCPs were in the range 0.26-7.38ng/mL [excluding musk xylene (MX) and Musk ketone (MK)] and 0.20-9.48ng/mL (excluding MX and MK) for bile samples with and without enzyme hydrolysis, respectively. After enzyme hydrolysis, 12 PCPs were detected in bile from fish collected from the Yangtze River, with a maximum detected concentration of 460ng/mL, for triclosan (TCS). The hydrolysis reaction indicated that high percentages of glucuronide and sulfate metabolites for some PCPs, i.e. four parabens and TCS, existed in the bile.
Determination and environmental risk assessment of synthetic musks in the water and sediments of the Jiaozhou Bay wetland, China.[Pubmed:29204937]
Environ Sci Pollut Res Int. 2018 Feb;25(5):4915-4923.
Human activity in estuarine areas has resulted in pollution of the aquatic environment, but little is known about the levels of synthetic musks (SMs) in river water and sediments in estuarine areas. This study investigated the concentrations and distribution of SMs in the Jiaozhou Bay wetland, including celestolide, phantolide, traseolide, galaxolide (HHCB), tonalide (AHTN), musk xylene and Musk ketone (MK). The SMs HHCB, AHTN and MK were detected at concentrations of 10.7-208, not detected (ND)-59.2 and ND-13.6 ng/L, respectively, in surface water samples and 13.1-27.3, 3.06-14.5 and 1.33-18.8 ng/g (dry weight; dw), respectively, in sediment samples. Based on the calculated total organic carbon (TOC) concentrations, there was no significant correlation between SMs and TOC in sediment samples (p > 0.05). The hazard quotients were 0.204, 0.386 and 0.059 for AHTN, HHCB and MK, respectively, which indicated no serious environmental impact, because these values are all less than 1. The concentrations of SMs decreased as the distance to the Xiaojianxi refuse landfill increased in both surface water and sediments. Compared with previous studies, the concentration of SMs in the Jiaozhou Bay wetland was relatively high. Therefore, more attention should be paid to SMs because of their persistent impact on human health and the environment.
Molecular mechanism of activation of human musk receptors OR5AN1 and OR1A1 by (R)-muscone and diverse other musk-smelling compounds.[Pubmed:29632183]
Proc Natl Acad Sci U S A. 2018 Apr 24;115(17):E3950-E3958.
Understanding olfaction at the molecular level is challenging due to the lack of crystallographic models of odorant receptors (ORs). To better understand the molecular mechanism of OR activation, we focused on chiral (R)-muscone and other musk-smelling odorants due to their great importance and widespread use in perfumery and traditional medicine, as well as environmental concerns associated with bioaccumulation of musks with estrogenic/antiestrogenic properties. We experimentally and computationally examined the activation of human receptors OR5AN1 and OR1A1, recently identified as specifically responding to musk compounds. OR5AN1 responds at nanomolar concentrations to Musk ketone and robustly to macrocyclic sulfoxides and fluorine-substituted macrocyclic ketones; OR1A1 responds only to nitromusks. Structural models of OR5AN1 and OR1A1 based on quantum mechanics/molecular mechanics (QM/MM) hybrid methods were validated through direct comparisons with activation profiles from site-directed mutagenesis experiments and analysis of binding energies for 35 musk-related odorants. The experimentally found chiral selectivity of OR5AN1 to (R)- over (S)-muscone was also computationally confirmed for muscone and fluorinated (R)-muscone analogs. Structural models show that OR5AN1, highly responsive to nitromusks over macrocyclic musks, stabilizes odorants by hydrogen bonding to Tyr260 of transmembrane alpha-helix 6 and hydrophobic interactions with surrounding aromatic residues Phe105, Phe194, and Phe207. The binding of OR1A1 to nitromusks is stabilized by hydrogen bonding to Tyr258 along with hydrophobic interactions with surrounding aromatic residues Tyr251 and Phe206. Hydrophobic/nonpolar and hydrogen bonding interactions contribute, respectively, 77% and 13% to the odorant binding affinities, as shown by an atom-based quantitative structure-activity relationship model.
Biomonitoring of chemicals in biota of two wetland protected areas exposed to different levels of environmental impact: results of the "PREVIENI" project.[Pubmed:28822013]
Environ Monit Assess. 2017 Aug 18;189(9):456.
The PREVIENI project (funded by the Ministry of Environment) investigated the exposure to endocrine disrupters in samples of human population and environmental biota in Italy. The environmental biomonitoring considered two Italian WWF Oasis, with the aim to compare the presence and effects of endocrine disruptors in organisms from two protected natural areas, respectively, upstream and downstream a chemical emission site. Chemical analysis of pollutants' tissue levels was made on tissues from earthworm, barbell, trout, and coot, selected as bioindicator organisms. The contaminants considered were as follows: the perfluorinated compounds perfuoroctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), polychlorinated biphenyls (PCBs 58 congeners), polybrominated diphenyl ethers (PBDEs, 13 congeners), polycyclic aromatic hydrocarbons (PAHs, 16 compounds), toxic trace elements, the phthalate di-2-ethylexyl phthalate (DEHP) and its primary metabolite, bisphenol A, synthetic musk compounds (musk xylene, Musk ketone, tonalide, and galaxolide), and p-nonylphenol. The analyses showed low concentrations of most pollutants in all species from both areas, compared to available literature; noticeable exceptions were the increases of DEHP's primary metabolite, PBDE, PAHs, Hg, and Pb in barbells, and of PCB and Cd in earthworms from the downstream area. The results showed the presence of endocrine disruptors, including those considered as "non-persistent," in bioindicators from protected areas, albeit at low levels. The results provide a contribution to the evaluation of reference values in biota from Mediterranean Europe and support the relevance of monitoring exposure to pollutants, in particular for freshwater environment, also in protected areas.
Sorption of three synthetic musks by microplastics.[Pubmed:28982477]
Mar Pollut Bull. 2018 Jan;126:606-609.
Microplastics and synthetic musks (SMs) are two typical organic pollutants in the marine environment. In this study, the sorption of three SMs to microplastics in a simulated seawater environment was examined. Tonalide (AHTN), musk xylene (MX), and Musk ketone (MK) were the musks investigated, while polypropylene (PP) was used as the microplastic. It was found that the equilibrium sorption time was about 10h and the adsorption kinetics model conformed to a Lagergren adsorption model. The adsorption capacity increased with decreasing particle size. Adsorption reached a peak at 25 degrees C, and the adsorption capacity was not sensitive to the concentration of sodium chloride. There is a need for more research and monitoring of microplastics in the marine environment due to their strong ability to absorb organic pollutants.


