MurracarpinCAS# 120786-76-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 120786-76-7 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
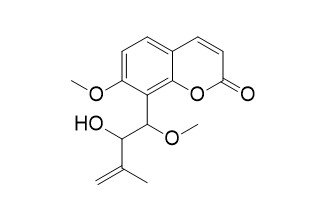
| Formula | C16H18O5 | M.Wt | 290.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Murracarpin Dilution Calculator

Murracarpin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4447 mL | 17.2236 mL | 34.4471 mL | 68.8942 mL | 86.1178 mL |
| 5 mM | 0.6889 mL | 3.4447 mL | 6.8894 mL | 13.7788 mL | 17.2236 mL |
| 10 mM | 0.3445 mL | 1.7224 mL | 3.4447 mL | 6.8894 mL | 8.6118 mL |
| 50 mM | 0.0689 mL | 0.3445 mL | 0.6889 mL | 1.3779 mL | 1.7224 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3445 mL | 0.6889 mL | 0.8612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Naringin 6''-acetate
Catalog No.:BCN0783
CAS No.:139934-60-4
- Erythro-Guaiacylglycerol-beta-coniferyl aldehyde ether
Catalog No.:BCN0782
CAS No.:74474-55-8
- 3',4',5',5,7-Pentamethoxyflavanone
Catalog No.:BCN0781
CAS No.:479672-30-5
- 6alpha-Hydroxydehydropachymic acid
Catalog No.:BCN0780
CAS No.:176390-67-3
- Quercetin 3,7-diglucoside
Catalog No.:BCN0779
CAS No.:6892-74-6
- (Z)-3,11-dimethy-7-methylene-9,14-epoxy-1,6,10-dodecatrien-3-ol
Catalog No.:BCN0778
CAS No.:1392202-57-1
- (2S)-5,7,3',4'-tetramethoxyflavanone
Catalog No.:BCN0777
CAS No.:74628-43-6
- 4'-Hydroxy-5,7,3'-trimethoxyflavone
Catalog No.:BCN0776
CAS No.:1239-68-5
- Tetillapyrone
Catalog No.:BCN0775
CAS No.:363136-43-0
- Lariciresinol 4'-O-glucoside
Catalog No.:BCN0774
CAS No.:107110-16-7
- 3'-Hydroxy-5,7,4'-trimethoxyflavone
Catalog No.:BCN0773
CAS No.:33554-52-8
- 4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No.:BCN0772
CAS No.:85644-03-7
- Melitidin
Catalog No.:BCN0785
CAS No.:1162664-58-5
- threo-Guaiacylglycerol-beta-coniferyl aldehyde ether
Catalog No.:BCN0786
CAS No.:650600-33-2
- Citrusin C
Catalog No.:BCN0787
CAS No.:18604-50-7
- Bergamjuicin
Catalog No.:BCN0788
CAS No.:2376307-20-7
- 3-Hydroxymollugin
Catalog No.:BCN0789
CAS No.:154706-45-3
- Narirutin 4'-glucoside
Catalog No.:BCN0790
CAS No.:17257-22-6
- Norwogonin-8-O-glucuronide
Catalog No.:BCN0791
CAS No.:118525-47-6
- 1,3,4,6-Tetragalloylglucose
Catalog No.:BCN0792
CAS No.:26922-99-6
- 2,11,12-Trihydroxy-7,20-epoxy-8,11,13-abietatriene
Catalog No.:BCN0793
CAS No.:1608462-12-9
- 4-O-Caffeoyl-3-O-syringoylquinic acid
Catalog No.:BCN0794
CAS No.:1207645-17-1
- Crepidiaside B
Catalog No.:BCN0795
CAS No.:101921-35-1
- Jacquilenin
Catalog No.:BCN0796
CAS No.:7726-34-3
Vasorelaxing activity of two coumarins from Murraya paniculata leaves.[Pubmed:24694618]
Biol Pharm Bull. 2014;37(4):694-7.
In the search for novel chemical scaffolds leading to potential antihypertensive agents, the methanol extract of Murraya paniculata leaves was assessed for its effects on isolated rat aorta rings. The vasorelaxing effect of the chloroform fraction of the methanol plant extract was the most potent for its vasorelaxing activity on rat aorta rings contracted by 60 mM K(+) (K60). Two coumarins were isolated from the chloroform fraction: the novel kimcuongin (1) and the known Murracarpin (2). Their structures were determined from spectroscopic evidences including (1)H- and (13)C-NMR, correlation spectroscopy (COSY), nuclear Overhauser effect spectroscopy (NOESY), heteronuclear multiple bond correlation (HMBC), heteronuclear single quantum correlation (HSQC), and high resolution mass spectrometry (HR-MS). Kimcuongin and, to a lesser extent, Murracarpin, showed vasorelaxing activity with IC50 values of 37.7 microM and 139.3 microM, respectively. The coumarins kimcuongin and Murracarpin may thus represent a novel class of vasodilators of natural source.
Evaluation of anti-inflammatory and antinociceptive activities of Murraya exotica.[Pubmed:20738224]
Pharm Biol. 2010 Dec;48(12):1344-53.
CONTEXT: Leaves of Murraya exotica L. (Rutaceae) are used for the treatment of various disorders such as cough, fever, and infectious wounds, as well as alleviating pains in folk medicine in southern China. OBJECTIVE: The objectives of this study were to investigate the in vivo antinociceptive and anti-inflammatory activities of ethanol (70%) extracts and isolated compounds obtained from the dried leaves of M. exotica. MATERIALS AND METHODS: The antinociceptive activities were evaluated with the methods of acetic acid-induced writhing response and hot-plate latent pain response test. Carrageenan induced hind paw edema, xylene induced ear edema, and a rat knee osteoarthritis model were employed to measure the anti-inflammatory activities. The compounds were isolated using column chromatography and thin-layer chromatography, and the structures identified by (1)H NMR, (1)(3)C NMR, MS, and IR. RESULTS: The ethanol (70%) extracts significantly decreased in the acetic acid-induced writhing response; increased in hot-plate latency; suppressed xylene induced ear swelling and the carrageenan-induced paw edema effectively. In the rat knee osteoarthritis model, the treatment of the ethanol (70%) extracts resulted in a significant increase in the activity of superoxide dismutase, an inhibition on inducible nitric oxide synthase activity, and a decrease in the contents of interleukin-1beta and tumor necrosis factor-alpha of the rat serum. Following this, we explored the components of the ethanol (70%) extracts and isolated six known coumarins, including Murracarpin, which exhibited the most potential in antinociceptive and anti-inflammatory activities. DISCUSSION AND CONCLUSION: M. exotica displayed remarkable antinociceptive and anti-inflammatory activities.


