MonomyristinCAS# 589-68-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 589-68-4 | SDF | Download SDF |
| PubChem ID | 79050 | Appearance | Powder |
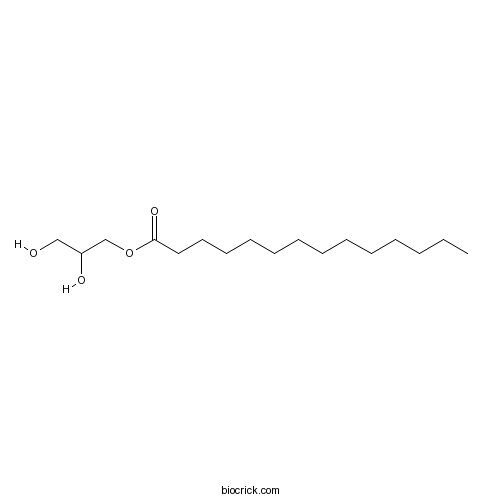
| Formula | C17H34O4 | M.Wt | 302.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 1-myristoyl glycerol;1-tetradecanoylglycerol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3-dihydroxypropyl tetradecanoate | ||
| SMILES | CCCCCCCCCCCCCC(=O)OCC(CO)O | ||
| Standard InChIKey | DCBSHORRWZKAKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H34O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-17(20)21-15-16(19)14-18/h16,18-19H,2-15H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Monomyristin Dilution Calculator

Monomyristin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3063 mL | 16.5317 mL | 33.0633 mL | 66.1266 mL | 82.6583 mL |
| 5 mM | 0.6613 mL | 3.3063 mL | 6.6127 mL | 13.2253 mL | 16.5317 mL |
| 10 mM | 0.3306 mL | 1.6532 mL | 3.3063 mL | 6.6127 mL | 8.2658 mL |
| 50 mM | 0.0661 mL | 0.3306 mL | 0.6613 mL | 1.3225 mL | 1.6532 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3306 mL | 0.6613 mL | 0.8266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nalmefene hydrochloride
Catalog No.:BCC7857
CAS No.:58895-64-0
- Arjungenin
Catalog No.:BCN8223
CAS No.:58880-25-4
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- Ophiopogonanone F
Catalog No.:BCN6409
CAS No.:588706-67-6
- Ophiopogonanone E
Catalog No.:BCN6625
CAS No.:588706-66-5
- 9-Oxonerolidol
Catalog No.:BCN5801
CAS No.:58865-88-6
- Secoxyloganin
Catalog No.:BCN5800
CAS No.:58822-47-2
- [Leu5]-Enkephalin
Catalog No.:BCC5831
CAS No.:58822-25-6
- SB 297006
Catalog No.:BCC6129
CAS No.:58816-69-6
- Toosendanin
Catalog No.:BCN1007
CAS No.:58812-37-6
- Benzalazine
Catalog No.:BCC8843
CAS No.:588-68-1
- KU 55933
Catalog No.:BCC2475
CAS No.:587871-26-9
- Laurolitsine
Catalog No.:BCN2634
CAS No.:5890-18-6
- Cassythicine
Catalog No.:BCN5802
CAS No.:5890-28-8
- Z-Glu-OtBu
Catalog No.:BCC2778
CAS No.:5891-45-2
- D-Phe-Ol
Catalog No.:BCC2580
CAS No.:58917-85-4
- Cyclizine 2HCl
Catalog No.:BCC4518
CAS No.:5897-18-7
- Bestatin
Catalog No.:BCC1221
CAS No.:58970-76-6
- Xanthurenic acid
Catalog No.:BCC7866
CAS No.:59-00-7
- DL-alpha-Tocopherol
Catalog No.:BCN2200
CAS No.:59-02-9
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
- Ethopabate
Catalog No.:BCC8964
CAS No.:59-06-3
- 5-BrdU
Catalog No.:BCC5293
CAS No.:59-14-3
- D-Galactose
Catalog No.:BCN8528
CAS No.:59-23-4
Absorption properties of micellar lipid metabolites into Caco2 cells.[Pubmed:17582542]
Lipids. 2007 Jul;42(7):613-9.
To elucidate the absorption characteristics of dietary lipids in the human intestine, we investigated the cellular uptake of lipid metabolites using a differential monolayer of the Caco2 cells. As lipid metabolites, several free fatty acids and 2-monoacylglycerols, were formed a mixed micelle by bile salts and lysophospholipids and they were supplied to the Caco2 cells. To estimate the effect of the mixed micelles on the permeability of cells' membranes during incubation with the mixed micelles, the transepitherial electrical resistance (TEER) value was monitored, and no pronounced changes of TEER was detected. This suggested that mixed micelles did not affect their cellular properties of the barrier measured by TEER. The lipid metabolites transferred from the mixed micelle into the Caco2 cells were determined quantitatively by an enzymatic colorimetric method and were done by thin layer chromatography (TLC) for a species of acylglycerols. These highly sensitive methods enabled us to monitor the transepithelial transports of various kinds of non-isotope-labeled various lipid metabolites. Newly re-synthesized triacylglycerols were accumulated in Caco2 cells after 30 min incubation with the mixed micelles, and their amounts increased gradually for 4 h. The secretion of re-esterified triacylglycerols into a basolateral medium from the Caco2 cells began at 2 h after the mixed micelles were added to the apical medium. The intake of external lipid metabolites by the Caco2 cells were evaluated by an initial 2-h incubation with the mixed micelles. For example, 2-Monomyristin and 2-monopalmitin were more rapidly transferred into the Caco2 cells from the mixed micelles than 2-monocaprin was. On the other hand, the absorption rates of capric acid, lauric acid and myristic acid by the cells were larger than those of stearic acid and oleic acid. It revealed that the side-chain structure of these lipid metabolites affected their absorption by the Caco2 cells. The results of this study suggested that the Caco2 cell monolayer could be a useful model for investigating the involvement of dietary lipids in the transepithelial absorption in the human intestine.
Structure of nonionic surfactant (glycerol alpha-monomyristate) micelles in organic solvents: a SAXS study.[Pubmed:19358555]
J Phys Chem B. 2009 May 7;113(18):6290-8.
In this paper we present the study of reverse micellar aggregates formed by glycerol alpha-monomyristate (C(14)G(1)) in different organic solvents. We have investigated the structure (mainly shape and size) of the Monomyristin reverse micelles depending on alkyl chain length of the oils, temperature, and the surfactant concentration. Moreover, we present how addition of trace polar additives such as water or glycerol and the hydrophilic size of the surfactant affect the structure of the micelles. Monomyristin could not form any self-organized structure in nonpolar media at room temperature because of its smaller headgroup size and tends to separate from the nonpolar media. In dilute regions this surfactant forms a solid dispersion, which upon heating transforms into an isotropic reverse micellar solution. For the structural characterization of the reverse micelles formed at elevated temperatures, we have performed small-angle X-ray scattering (SAXS) measurements, and the data were analyzed utilizing the generalized indirect Fourier transformation (GIFT) method. The SAXS data have shown that the size of the reverse micelles increases with the alkyl chain length of oils and surfactant concentration but decreases with temperature. A dramatic growth to the micelles could be achieved when the hydrophilic size of the surfactant is increased. Furthermore, addition of trace water or glycerol induces a significant change in the structure of micelles in terms of a cross-section and maximum length. The added water tends to form a water pool in the micellar core and results in swollen micelles. Model-free cross-section analysis of the SAXS data provided complementary information on the structural parameters. Thus, the present study has highlighted the possible ways of controlling the structure, mainly the shape and the size of the inverse micelles.
Influence of the degree of unsaturation of the acyl side chain upon the interaction of analogues of 1-arachidonoylglycerol with monoacylglycerol lipase and fatty acid amide hydrolase.[Pubmed:16181610]
Biochem Biophys Res Commun. 2005 Nov 11;337(1):104-9.
Little is known as to the structural requirements of the acyl side chain for interaction of acylglycerols with monoacylglycerol lipase (MAGL), the enzyme chiefly responsible for the metabolism of the endocannabinoid 2-arachidonoylglycerol (2-AG) in the brain. In the present study, a series of twelve analogues of 1-AG (the more stable regioisomer of 2-AG) were investigated with respect to their ability to inhibit the metabolism of 2-oleoylglycerol by cytosolic and membrane-bound MAGL. In addition, the ability of the compounds to inhibit the hydrolysis of anandamide by fatty acid amide hydrolase (FAAH) was investigated. For cytosolic MAGL, compounds with 20 carbon atoms in the acyl chain and 2-5 unsaturated bonds inhibited the hydrolysis of 2-oleoylglycerol with similar potencies (IC50 values in the range 5.1-8.2 microM), whereas the two compounds with a single unsaturated bond were less potent (IC50 values 19 and 21 microM). The fully saturated analogue 1-monoarachidin did not inhibit the enzyme, whereas the lower side chain analogues 1-monopalmitin and 1-Monomyristin inhibited the enzyme with IC50 values of 12 and 32 microM, respectively. The 22-carbon chain analogue of 1-AG was also potent (IC50 value 4.5 microM). Introduction of an alpha-methyl group for the C20:4, C20:3, and C22:4 compounds did not affect potency in a consistent manner. For the FAAH and the membrane-bound MAGL, there was no obvious relationship between the degree of unsaturation of the acyl side chain and the ability to inhibit the enzymes. It is concluded that increasing the number of unsaturated bonds on the acyl side chain of 1-AG from 1 to 5 has little effect on the affinity of acylglycerols for cytosolic MAGL.
Adsorption of 1-Monoglycerides at the Hexane/Water Interface.[Pubmed:9878150]
J Colloid Interface Sci. 1999 Jan 1;209(1):173-178.
The interfacial tension of a hexane solution of 1-Monomyristin against water was measured as a function of temperature and concentration under atmospheric pressure. The interfacial tension decreases after adding an extremely small amount of 1-Monomyristin. The thermodynamic quantity changes associated with the adsorption were evaluated by applying the thermodynamic relations. The adsorbed film of 1-Monomyristin exhibits the expanded state on its interfacial pressure vs area curve under this experimental condition. The large negative value of the entropy change at high concentration is related to the restricted orientation of the polar head group of 1-Monomyristin at the hexane/water interface. We conclude that the entropy change for the system of 1-Monomyristin is smaller than that of tetradecanol because of a greater interaction between the large hydrophilic group of 1-Monomyristin and water molecules. An explanation is also given about the difference in energy change for both the systems: the adsorbed film of 1-Monomyristin is more stabilized energetically than that of tetradecanol by the greater interaction with water molecules. Copyright 1999 Academic Press.
Preparation and Characterization of SN-38-Encapsulated Phytantriol Cubosomes Containing alpha-Monoglyceride Additives.[Pubmed:27250792]
Chem Pharm Bull (Tokyo). 2016;64(6):577-84.
SN-38 is a potent active metabolite of irinotecan that has been considered as an anticancer candidate. However, the clinical development of this compound has been hampered by its poor aqueous solubility and chemical instability. In this study, we developed SN-38-encapsulated cubosomes to resolve these problems. Six alpha-monoglyceride additives, comprising monocaprylin, monocaprin, monolaurin, Monomyristin, monopalmitin, and monostearin, were used to prepare phytantriol (PHYT) cubosomes by probe sonication. The mean particle size, polydispersity index, and zeta potential values of these systems were around 190-230 nm, 0.19-0.25 and -17 to -22 mV, respectively. Small-angle X-ray scattering analyses confirmed that the SN-38-encapsulated cubosomes existed in the Pn3m space group both with and without the additives. The monoglyceride additives led to around a two-fold increase in the solubility of SN-38 compared with the PHYT cubosome. The drug entrapment efficiency of PHYT cubosomes with additives was greater than 97%. The results of a stability study at 25 degrees C showed no dramatic changes in the particle size or polydispersity index characteristics, with at least 85% of the SN-38 existing in its active lactone form after 10 d, demonstrating the high stability of the cubosome nanoparticles. Furthermore, approximately 55% of SN-38 was slowly released from the cubosomes with additives over 96 h in vitro under physiological conditions. Taken together, these results show that the SN-38-encapsulated PHYT cubosome particles are promising drug carriers that should be considered for further in vivo experiments, including drug delivery to tumor cells using the enhanced permeability and retention effect.
Enzymatic synthesis of medium chain monoglycerides in a solvent-free system.[Pubmed:12018319]
Appl Biochem Biotechnol. 2002 Spring;98-100:987-96.
The synthesis of monocaprin, monolaurin, and Monomyristin in a solvent-free system was conducted by mixing a commercial immobilized lipase with the organic reactants (glycerol and fatty acids) in a 20-mL batch reactor with constant stirring. The effects of temperature, fatty acid/glycerol molar ratio, and enzyme concentration on the reaction conversion were determined. The addition of molecular sieves in the assays of Monomyristin synthesis was also evaluated. The reactions were carried out for 5 to 6 h and the nonpolar phase was analyzed by gas chromatography. The best results in terms of selectivity and conversion (defined as the percentage of fatty acid consumed) were achieved when the stoichiometric amount of reagents (molar ratio = 1) and 9% (w/w) commercial enzyme were used and the reaction was performed at 60 degrees C. The addition of molecular sieves did not improve the synthesis of Monomyristin. Conversions as high as 80%, with monoglycerides being the major products, were attained. After 5 h of reaction, the concentration of monoglyceride was about twice that of diglyceride, and only trace amounts of triglyceride were found. The results illustrate the technical possibility of producing medium chain monoglycerides in a solvent-free medium using a simple batch reactor.
Adsorption of 1-Monoglycerides at the Hexane/Water Interface.[Pubmed:10607455]
J Colloid Interface Sci. 1999 Dec 15;220(2):374-379.
The interfacial tension of a hexane solution of 1-monolaurin against water was measured as a function of temperature and concentration under atmospheric pressure. The thermodynamic quantity changes associated with the adsorption of 1-monolaurin were evaluated and compared with those of the previously reported 1-Monomyristin. The decrease of two carbon atoms in the hydrocarbon chain results in a slight expansion of the 1-monolaurin adsorbed film and in a slight decrease in entropy and energy changes compared with those of the 1-Monomyristin system. The large negative value of the entropy change at a high concentration is related to the restricted orientation of the polar head group of 1-monolaurin at the hexane/water interface due to the strong interaction between the large hydrophilic group of 1-monolaurin and the water molecules, as in the 1-Monomyristin system. The origin of the distinction in the entropy change behavior between the adsorption from the hexane phase and water phase was discussed. The usefulness of an easier calculation process for the partial molar entropy change is verified by comparison with the usual reliable value and with the entropy of adsorption. Copyright 1999 Academic Press.
Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships.[Pubmed:19469285]
Pol J Microbiol. 2009;58(1):43-7.
The antibacterial activity of the medium chain fatty acids and their 1-monoglycerides was evaluated towards several Gram-positive strains belonging to the genera Staphylococcus, Corynebacterium, Bacillus, Listeria and Streptococcus. The 1-monoglycerides were more active than the fatty acids with monolaurin being the most active compound. Interesting effects were observed when the streptococcal strain Streptococcus pyogenes was used as a test microorganism. First, blocking of the hydroxyl groups of the glycerol moiety of monolaurin led to a compound with remarkable antibacterial activity (MIC, 3.9 microg/ml). Secondly, synergistic relationships were observed between monolaurin and monocaprin as well as between monolaurin and the poorly active lauric acid when their two component mixtures were examined. The mixtures in which one of the components was 2-fold more predominant than the other one were much more active than the pure components taken individually. Moreover, the presence of the components in ratio 1:1 was disadvantageous. Synergistic relationships were also found between monolaurin and Monomyristin towards Staphylococcus aureus 209 when Monomyristin was in the same quantity as monolaurin or in shortage.


