MikaninCAS# 4324-53-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4324-53-2 | SDF | Download SDF |
| PubChem ID | 15560536 | Appearance | Yellow powder |
| Formula | C18H16O7 | M.Wt | 344.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
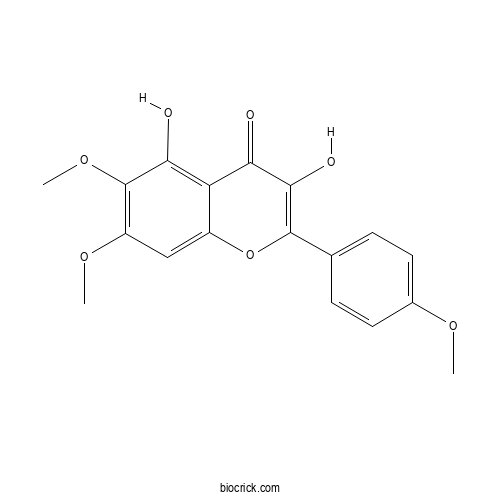
| Chemical Name | 3,5-dihydroxy-6,7-dimethoxy-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=C(C(=O)C3=C(C(=C(C=C3O2)OC)OC)O)O | ||
| Standard InChIKey | SGCYGSKAUSOJND-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O7/c1-22-10-6-4-9(5-7-10)17-16(21)14(19)13-11(25-17)8-12(23-2)18(24-3)15(13)20/h4-8,20-21H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mikanin Dilution Calculator

Mikanin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9044 mL | 14.5222 mL | 29.0444 mL | 58.0889 mL | 72.6111 mL |
| 5 mM | 0.5809 mL | 2.9044 mL | 5.8089 mL | 11.6178 mL | 14.5222 mL |
| 10 mM | 0.2904 mL | 1.4522 mL | 2.9044 mL | 5.8089 mL | 7.2611 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1618 mL | 1.4522 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercitrin 2''-O-arabinoside
Catalog No.:BCN0678
CAS No.:104683-55-8
- Volkensiflavone
Catalog No.:BCN0677
CAS No.:27542-37-6
- 5,8-Dihydroxy-6,7-dimethoxyflavone
Catalog No.:BCN0676
CAS No.:73202-52-5
- Nuchensin
Catalog No.:BCN0675
CAS No.:25782-24-5
- 2'-Hydroxyneophellamuretin
Catalog No.:BCN0674
CAS No.:1396769-20-2
- 6-Methoxykaempferol 3-O-glucoside
Catalog No.:BCN0673
CAS No.:63422-27-5
- 6-Methoxykaempferol
Catalog No.:BCN0672
CAS No.:32520-55-1
- 5,6-Dihydroxy-3,7,4'-trimethoxyflavone
Catalog No.:BCN0671
CAS No.:84019-17-0
- 5,6-Dihydroxy-3,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0670
CAS No.:63296-15-1
- 3,5,8,3',4',5'-Hexamethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN0669
CAS No.:82668-95-9
- 7,4'-Dihydroxy-6,8-diprenylflavanone
Catalog No.:BCN0668
CAS No.:50939-03-2
- Isosativanone
Catalog No.:BCN0667
CAS No.:82829-55-8
- 6'-Prenylisorhamnetin
Catalog No.:BCN0680
CAS No.:1859979-02-4
- 2'-Prenylisorhamnetin
Catalog No.:BCN0681
CAS No.:1932668-04-6
- Orientin 2''-O-rhamnoside
Catalog No.:BCN0682
CAS No.:81398-30-3
- Naringenin 7-O-β-D-glucuronide methyl ester
Catalog No.:BCN0683
CAS No.:1985597-72-5
- Eriodictyol 7-O-β-D-glucuronide ethyl ester
Catalog No.:BCN0684
CAS No.:847025-48-3
- 8-(1,1-Dimethyl-2-propenyl)-3'-methoxykaempferol
Catalog No.:BCN0685
CAS No.:1859979-00-2
- Eriodictyol 7-O-methylglucuronide
Catalog No.:BCN0686
CAS No.:133360-42-6
- 8-(1,1-Dimethyl-2-propenyl)kaempferol
Catalog No.:BCN0687
CAS No.:142646-43-3
- 3,5,7-Trihydroxy-8-methoxyflavone
Catalog No.:BCN0688
CAS No.:5928-42-7
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
- Platanoside
Catalog No.:BCN0690
CAS No.:133740-25-7
- Morelloflavone
Catalog No.:BCN0691
CAS No.:16851-21-1
Isolation of compound and CNS depressant activities of Mikania scandens Willd with special emphasis to brain biogenic amines in mice.[Pubmed:25651612]
Indian J Exp Biol. 2014 Dec;52(12):1186-94.
Mikania scandens, a twining herb that grows as a weed in India and Bangladesh is used as vegetables and is a good source of vitamin A, C, B complex, Mikanin, sesquiterpenes, betasitosterin, stigmasterol and friedelin. The present communication reports CNS depressant activities with special emphasis to brain biogenic amines in mice. Ethanol extract of leaves of M. scandens (EEMS) was prepared by Soxhalation and analyzed chemically. EEMS potentiated sleeping time induced by pentobarbitone, diazepam and meprobamate and showed significant reduction in the number of writhes and stretches. EEMS caused significant protection against pentylene tetrazole-induced convulsion and increased catecholamines and brain amino acids level significantly. Results showed that EEMS produced good CNS depressant effects in mice.
Phenolic constituents from the roots of Mikania micrantha and their allelopathic effects.[Pubmed:23822807]
J Agric Food Chem. 2013 Jul 31;61(30):7309-14.
Four new thymol derivatives, 8,10-dihydroxy-9-benzoyloxythymol (1), 9-isobutyryloxy-10-hydroxythymol (2), 7,8,9,10-tetrahydroxythymol (3), and 7,8,10-trihydroxy-9-E-feruloyloxythymol (4), were isolated from the fresh roots of Mikania micrantha , along with 8,9,10-trihydroxythymol (5), 8,10-dihydroxy-9-acetoxythymol (6), 8,10-dihydroxy-9-isobutyryloxythymol (7), 8,10-dihydroxy-9-(2-methylbutyryloxy)thymol (8), 8,9-dehydro-10-hydroxythymol (9), 8-methoxy-9-hydroxythymol (10), ethyl caffeate (11), ethyl ferulate (12), 3,5-di-O-caffeoylquinic acid (13), and Mikanin (14). Their structures were determined by spectroscopic methods. The known thymol derivatives (5-10) were obtained from the genus Mikania for the first time. Allelopathic effects of these compounds on Arabidopsis thaliana seeds were evaluated by a filter paper assay. After the treatment at 0.1 mM for 4 days, the seed germination rate with compound 8 was 48% and the inhibitory rates of shoot growth with compounds 1, 2, 7-10, and 12 were over 40%. The IC50 values of compounds 1 and 8 on shoot growth were 342.5 and 625 muM, respectively.
Antiviral constituents against respiratory viruses from Mikania micrantha.[Pubmed:19267453]
J Nat Prod. 2009 May 22;72(5):925-8.
Phytochemical investigation of the dried aerial parts of Mikania micrantha led to the isolation of a new sesquiterpene, 3beta-acetoxy-1,10-epoxy-4-germacrene-12,8;15,6-diolide (1), along with six known constituents: 1,10-epoxy-4-germacrene-12,8;15,6-diolide (2), dihydromikanolide (3), potassium Mikanin 3-sulfate (4), Mikanin (5), alpinetin (6), and ergosta-7,22-dien-3beta-ol (7). Their structures were elucidated by spectroscopic methods, and the molecular structures and stereochemistry of sesquiterpene lactones 1-3 were revealed by single-crystal X-ray analysis. Compound 2 showed moderate activity against respiratory syncytial virus (IC(50) = 37.4 uM) and parainfluenza type 3 virus (IC(50) = 37.4 uM) with a therapeutic index (TI) of 16.0 for both compounds. Compound 4, the main component of M. micrantha, exhibited inhibitory activity against parainfluenza type 3 virus with IC(50) (19.7 uM) and TI (24.0) values comparable to those of ribavirin, serving as a positive control.
A novel 1:1 complex of potassium mikanin-3-O-sulfate with methanol.[Pubmed:11558604]
Chem Pharm Bull (Tokyo). 2001 Sep;49(9):1166-9.
Mikanin-3-O-sulfate (1), in the form of its potassium salt, together with Mikanin (2) and alpinetin (3) were isolated from Mikania micrantha. The crystal structures of K(1) x CH3OH, 2 and 3 x H2O were established by X-ray crystallography. The potassium ions in K(1) x CHO3H are bridged by O5, O7 and O8 to form a chain of face-sharing KO8 coordination polyhedra, from which the aglycon units are outstretched to form a polymeric molecular column. Adjacent molecular columns are linked by pi-pi stacking between parallel, intercalating aglycon units to form layers matching the (101) family of planes, which are further interconnected into a three-dimensional supramolecular assembly. Sulfation at 3-OH induced better co-planarity and conjugation of the rings.


