Methylgomisin OCAS# 1276654-07-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1276654-07-9 | SDF | Download SDF |
| PubChem ID | 46192500 | Appearance | Powder |
| Formula | C24H30O7 | M.Wt | 430.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
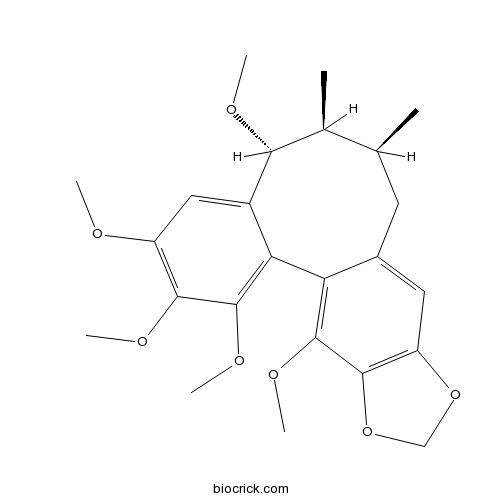
| Chemical Name | (8R,9S,10S)-3,4,5,8,19-pentamethoxy-9,10-dimethyl-15,17-dioxatetracyclo[10.7.0.02,7.014,18]nonadeca-1(19),2,4,6,12,14(18)-hexaene | ||
| SMILES | CC1CC2=CC3=C(C(=C2C4=C(C(=C(C=C4C(C1C)OC)OC)OC)OC)OC)OCO3 | ||
| Standard InChIKey | FCFAWFPHAOTXPJ-KQHSUYLTSA-N | ||
| Standard InChI | InChI=1S/C24H30O7/c1-12-8-14-9-17-22(31-11-30-17)23(28-6)18(14)19-15(20(26-4)13(12)2)10-16(25-3)21(27-5)24(19)29-7/h9-10,12-13,20H,8,11H2,1-7H3/t12-,13-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methylgomisin O has anti- inflammatory activity, it -induced attenuation of inflammatory cytokine production upon exposure to LPS was at least partially mediated by the suppression of NF-κB and MAPK pathway. Methylgomisin O exhibits strong cytotoxic effects on HL-60. It exhibits considerable inhibitory activity against tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6). |
| Targets | NF-κB | MAPK | TNF-α | IL Recepter |
| In vitro | Anti-inflammatory effect and mechanism of methylgomisin O.[Reference: WebLink]Chinese journal of veterinary science, 2011, 31(9):1309-1308.Methylgomisin O was a new dibenzocyclooctadiene lignan isolated from the fruits of Schisandra sphenanthera.
The Antiproliferative Effects of Compounds Isolated from Schisandra chinensis.[Reference: WebLink]Korean journal of food science & technology, 2014, 46(6):665-670.We isolated twelve lignans and three terpenoids were isolated from the n-hexane fraction of Schisandra chinensis extract. New Dibenzocyclooctadiene Lignans from Schisandra sphenanthera and Their Proinflammatory Cytokine Inhibitory Activities.[Reference: WebLink]Zeitschrift Für Naturforschung B, 2010, 65(2):1-8.
|

Methylgomisin O Dilution Calculator

Methylgomisin O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3229 mL | 11.6144 mL | 23.2288 mL | 46.4576 mL | 58.072 mL |
| 5 mM | 0.4646 mL | 2.3229 mL | 4.6458 mL | 9.2915 mL | 11.6144 mL |
| 10 mM | 0.2323 mL | 1.1614 mL | 2.3229 mL | 4.6458 mL | 5.8072 mL |
| 50 mM | 0.0465 mL | 0.2323 mL | 0.4646 mL | 0.9292 mL | 1.1614 mL |
| 100 mM | 0.0232 mL | 0.1161 mL | 0.2323 mL | 0.4646 mL | 0.5807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dichotomine B
Catalog No.:BCN8913
CAS No.:755036-41-0
- 2-Methoxy-5-acetoxy-furanogermacr-1(10)-en-6-one
Catalog No.:BCN8912
CAS No.:1809980-25-3
- Methyl neochebulinate
Catalog No.:BCN8911
CAS No.:1236310-34-1
- Biatractylolide
Catalog No.:BCN8910
CAS No.:182426-37-5
- Arisantetralone B
Catalog No.:BCN8909
CAS No.:1161947-96-1
- Rebaudioside F
Catalog No.:BCN8908
CAS No.:438045-89-7
- Arisanschinin E
Catalog No.:BCN8907
CAS No.:1333378-33-8
- Isolappaol C
Catalog No.:BCN8906
CAS No.:929905-15-7
- Stevioside D
Catalog No.:BCN8905
CAS No.:1310055-59-4
- Nortrachelogenin-8'-O-beta-glucoside
Catalog No.:BCN8904
CAS No.:858127-38-5
- 3-Acetyl-ginsenoside F1
Catalog No.:BCN8903
CAS No.:1881225-08-6
- (E)-6-O-(p-coumaroyl)scandoside methyl ester
Catalog No.:BCN8902
CAS No.:83946-90-1
- Stevia impurity (13-[(2-O-6-deoxy-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]ent-kaur-16-en-19-oic acid β-D-glucopyranosyl ester)
Catalog No.:BCN8915
CAS No.:1309929-72-3
- Schisphenin E
Catalog No.:BCN8916
CAS No.:1311376-52-9
- Licoflavanone
Catalog No.:BCN8917
CAS No.:119240-82-3
- Isoeuphorbetin
Catalog No.:BCN8918
CAS No.:50677-55-9
- 3-Hydroxy-4',5-dimethoxystilbene
Catalog No.:BCN8919
CAS No.:58436-29-6
- Ginsenoside MC
Catalog No.:BCN8920
CAS No.:175484-06-7
- (1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No.:BCN8921
CAS No.:1393342-06-7
- 5-Hydroxy-7,8-dimethoxy (2R)-flavanone-5-O-beta-D-glucopyranoside
Catalog No.:BCN8922
CAS No.:942626-74-6
- (3S,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8923
CAS No.:87095-75-8
- (3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8924
CAS No.:232261-31-3
- Nardoaristolone B
Catalog No.:BCN8925
CAS No.:1422517-82-5
- 19-O-beta-D-carboxyglucopyranosyl-12-O-beta-D-glucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
Catalog No.:BCN8926
CAS No.:1011714-20-7
Isolation and anti-hepatitis B virus activity of dibenzocyclooctadiene lignans from the fruits of Schisandra chinensis.[Pubmed:25882501]
Phytochemistry. 2015 Aug;116:253-261.
Seven lignans with a dibenzocyclooctadiene skeleton, termed schinlignans A-G, and a 6,7-seco-homolignan, schischinone, together with seven known lignans, were isolated from the fruits of Schisandra chinensis (Turcz.) Baill. Their structures were elucidated by extensive spectroscopic methods, including HRESIMS, IR, UV, and 2D NMR (COSY, HMQC, COSY, and HMBC experiments). The stereochemistry at the chiral centers and the biphenyl moiety, were determined using ROESY, as well as via interpretation of their ECD spectra. Schinlignan G and Methylgomisin O exhibited potent anti-hepatitis B virus activity against HBV DNA replication with IC50 values of 5.13 and 5.49mugmL(-1), respectively.


