Methyl ganoderate C6CAS# 105742-81-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 105742-81-2 | SDF | Download SDF |
| PubChem ID | 102004758 | Appearance | Powder |
| Formula | C31H44O8 | M.Wt | 544.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
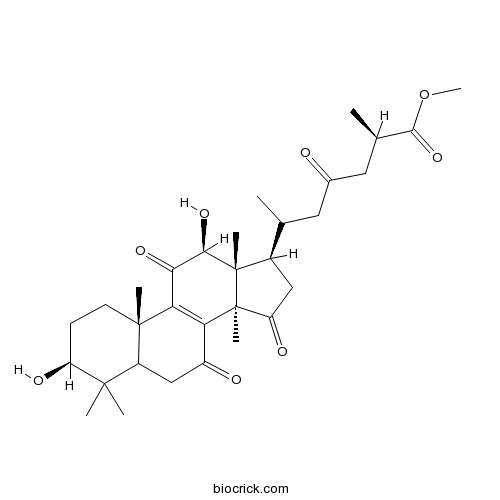
| Chemical Name | methyl (2R)-6-[(3S,10S,12S,13R,14R,17R)-3,12-dihydroxy-4,4,10,13,14-pentamethyl-7,11,15-trioxo-1,2,3,5,6,12,16,17-octahydrocyclopenta[a]phenanthren-17-yl]-2-methyl-4-oxoheptanoate | ||
| SMILES | CC(CC(=O)CC(C)C(=O)OC)C1CC(=O)C2(C1(C(C(=O)C3=C2C(=O)CC4C3(CCC(C4(C)C)O)C)O)C)C | ||
| Standard InChIKey | AZCYOYBNOUOOJJ-CGVPIJHZSA-N | ||
| Standard InChI | InChI=1S/C31H44O8/c1-15(11-17(32)12-16(2)27(38)39-8)18-13-22(35)31(7)23-19(33)14-20-28(3,4)21(34)9-10-29(20,5)24(23)25(36)26(37)30(18,31)6/h15-16,18,20-21,26,34,37H,9-14H2,1-8H3/t15?,16-,18-,20?,21+,26-,29+,30+,31+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl ganoderate C6 Dilution Calculator

Methyl ganoderate C6 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8359 mL | 9.1794 mL | 18.3587 mL | 36.7175 mL | 45.8968 mL |
| 5 mM | 0.3672 mL | 1.8359 mL | 3.6717 mL | 7.3435 mL | 9.1794 mL |
| 10 mM | 0.1836 mL | 0.9179 mL | 1.8359 mL | 3.6717 mL | 4.5897 mL |
| 50 mM | 0.0367 mL | 0.1836 mL | 0.3672 mL | 0.7343 mL | 0.9179 mL |
| 100 mM | 0.0184 mL | 0.0918 mL | 0.1836 mL | 0.3672 mL | 0.459 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganoderic acid C6
Catalog No.:BCN3257
CAS No.:105742-76-5
- AL 8697
Catalog No.:BCC8037
CAS No.:1057394-06-5
- Androstanolone 17-benzoate
Catalog No.:BCC8825
CAS No.:1057-07-4
- AT13148
Catalog No.:BCC5360
CAS No.:1056901-62-2
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- Mizolastine dihydrochloride
Catalog No.:BCC4132
CAS No.:1056596-82-7
- ML 9 hydrochloride
Catalog No.:BCC6644
CAS No.:105637-50-1
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- Fasudil (HA-1077) HCl
Catalog No.:BCC2542
CAS No.:105628-07-7
- Prostephanaberrine
Catalog No.:BCN4736
CAS No.:105608-27-3
- BMY 14802 hydrochloride
Catalog No.:BCC5759
CAS No.:105565-55-7
- Fmoc-Ser(tBu)-OPfp
Catalog No.:BCC3545
CAS No.:105751-13-1
- Sitostenone
Catalog No.:BCN5868
CAS No.:1058-61-3
- E-3810
Catalog No.:BCC1541
CAS No.:1058137-23-7
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- TSTU
Catalog No.:BCC2828
CAS No.:105832-38-0
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
Evaluation of N-[(11)C]methyl-AMD3465 as a PET tracer for imaging of CXCR4 receptor expression in a C6 glioma tumor model.[Pubmed:25094028]
Mol Pharm. 2014 Nov 3;11(11):3810-7.
The chemokine receptor CXCR4 and its ligand CXCL12 play an important role in tumor progression and metastasis. CXCR4 receptors are expressed by many cancer types and provide a potential target for treatment. Noninvasive detection of CXCR4 may aid diagnosis and improve therapy selection. It has been demonstrated in preclinical studies that positron emission tomography (PET) with a radiolabeled small molecule could enable noninvasive monitoring of CXCR4 expression. Here, we prepared N-[(11)C]methyl-AMD3465 as a new PET tracer for CXCR4. N-[(11)C]Methyl-AMD3465 was readily prepared by N-methylation with [(11)C]CH3OTf. The tracer was obtained in a 60 +/- 2% yield (decay corrected), the purity of the tracer was >99%, and specific activity was 47 +/- 14 GBq/mumol. Tracer stability was tested in vitro using liver microsomes and rat plasma; excellent stability was observed. The tracer was evaluated in rat C6 glioma and human PC-3 cell lines. In vitro cellular uptake of N-[(11)C]methyl-AMD3465 was receptor mediated. The effect of transition metal ions (Cu(2+), Ni(2+), and Zn(2+)) on cellular binding was examined in C6 cells, and the presence of these ions increased the cellular binding of the tracer 9-, 7-, and 3-fold, respectively. Ex vivo biodistribution and PET imaging of N-[(11)C]methyl-AMD3465 were performed in rats with C6 tumor xenografts. Both PET and biodistribution studies demonstrated specific accumulation of the tracer in the tumor (SUV 0.6 +/- 0.2) and other CXCR4 expressing organs, such as lymph node (1.5 +/- 0.2), liver (8.9 +/- 1.0), bone marrow (1.0 +/- 0.3), and spleen (1.0 +/- 0.1). Tumor uptake was significantly reduced (66%, p < 0.01) after pretreatment with Plerixafor (AMD3100). Biodistribution data indicates a tumor-to-muscle ratio of 7.85 and tumor-to-plasma ratio of 1.14, at 60 min after tracer injection. Our data demonstrated that N-[(11)C]methyl-AMD3465 is capable of detecting physiologic CXCR4 expression in tumors and other CXCR4 expressing tissues. These results warrant further evaluation of N-[(11)C]methyl-AMD3465 as a potential PET tracer for CXCR4 receptor imaging.
Enhancement of the Power Conversion Efficiency in the Inverted Organic Solar Cells Fabricated Utilizing a CeO2 Interlayer Between the Poly(3-hexylthiophene) (P3HT):[6,6]-Phenyl C6 Butyric Acid Methyl Ester and the Cathode.[Pubmed:26328337]
J Nanosci Nanotechnol. 2015 Jan;15(1):232-5.
CeO2 nanoparticles were synthesized by using a precipitation method. High-resolution transmission electron microscopy images, X-ray diffraction patterns, energy dispersive X-ray spectroscopy spectra, and UV-Visible absorption spectroscopy spectra showed that the formed samples were CeO2 polycrystalline nanoparticles. Inverted organic solar cells with a structure of indium-tin-oxide/CeO2/poly(3-hexylthiophene) (P3HT):[6,6]-phenyl C61 butyric acid methyl ester (PCBM)/MoO3/Ag were fabricated. Current density-voltage results showed that the power conversion efficiency of the device of the fabricated inverted OPV cells with a CeO2 interlayer between the P3HT:PCBM and the cathode was 0.39% larger than that without a CeO2 interlayer.
(13)C-Labeled Idohexopyranosyl Rings: Effects of Methyl Glycosidation and C6 Oxidation on Ring Conformational Equilibria.[Pubmed:28006104]
J Org Chem. 2017 Feb 3;82(3):1356-1370.
An ensemble of JHH, JCH, and JCC values was measured in aqueous solutions of methyl alpha- and beta-d-idohexopyranosides containing selective (13)C-enrichment at various carbons. By comparing these J-couplings to those reported previously in the alpha- and beta-d-idohexopyranoses, methyl glycosidation was found to affect ring conformational equilibria, with the percentages of (4)C1 forms based on (3)JHH analysis as follows: alpha-d-idopyranose, approximately 18%; methyl alpha-d-idopyranoside, approximately 42%; methyl beta-d-idopyranoside, approximately 74%; beta-d-idopyranose, 82%. JCH and JCC values were analyzed with assistance from theoretical values obtained from density functional theory (DFT) calculations. Linearized plots of the percentages of (4)C1 against limiting JCH and JCC values in the chair forms were used to (a) determine the compatibility of the experimental JCH and JCC values with (4)C1/(1)C4 ratios determined from JHH analysis and (b) determine the sensitivity of specific JCH and JCC values to ring conformation. Ring conformational equilibria for methyl idohexopyranosides differ significantly from those predicted from recent molecular dynamics (MD) simulations, indicating that equilibria determined by MD for ring configurations with energetically flat pseudorotational itineraries may not be quantitative. J-couplings in methyl alpha-l-[6-(13)C]idopyranosiduronic acid and methyl alpha-d-[6-(13)C]glucopyranosiduronic acid were measured as a function of solution pH. The ring conformational equilibrium is pH-dependent in the iduronic acid.
Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake.[Pubmed:23727015]
Neurotoxicology. 2013 Sep;38:1-8.
Various forms of mercury possess different rates of absorption, metabolism and excretion, and consequently, toxicity. Methylmercury (MeHg) is a highly neurotoxic organic mercurial. Human exposure is mostly due to ingestion of contaminated fish. Ethylmercury (EtHg), another organic mercury compound, has received significant toxicological attention due to its presence in thimerosal-containing vaccines. This study was designed to compare the toxicities induced by MeHg and EtHg, as well as by their complexes with cysteine (MeHg-S-Cys and EtHg-S-Cys) in the C6 rat glioma cell line. MeHg and EtHg caused significant (p<0.0001) decreases in cellular viability when cells were treated during 30min with each mercurial following by a washing period of 24h (EC50 values of 4.83 and 5.05muM, respectively). Significant cytotoxicity (p<0.0001) was also observed when cells were treated under the same conditions with MeHg-S-Cys and EtHg-S-Cys, but the respective EC50 values were significantly increased (11.2 and 9.37muM). l-Methionine, a substrate for the l-type neutral amino acid carrier transport (LAT) system, significantly protected against the toxicities induced by both complexes (MeHg-S-Cys and EtHg-S-Cys). However, no protective effects of l-methionine were observed against MeHg and EtHg toxicities. Corroborating these findings, l-methionine significantly decreased mercurial uptake when cells were exposed to MeHg-S-Cys (p=0.028) and EtHg-S-Cys (p=0.023), but not to MeHg and EtHg. These results indicate that the uptake of MeHg-S-Cys and EtHg-S-Cys into C6 cells is mediated, at least in part, through the LAT system, but MeHg and EtHg enter C6 cells by mechanisms other than LAT system.
Introduction of Methyl Groups at C2 and C6 Positions Enhances the Antiangiogenesis Activity of Curcumin.[Pubmed:26391485]
Sci Rep. 2015 Sep 22;5:14205.
Curcumin has diverse biological activities, but is known to undergo rapid metabolism via reduction of vinylic double bonds and phase II conjugation. To prevent reductive metabolism of curcumin, we introduced a methyl group at both C2 and C6 positions (compound 1) or at the C2 position (compound 2) of curcumin, creating steric hindrance on double bonds against metabolizing enzymes. As predicted, these compounds were resistant to reduction by alcohol dehydrogenase. Compound 1 was further evaluated for its antiangiogenesis activity in vitro and in vivo. It exhibited significantly greater inhibitory activity than curcumin against endothelial cell migration, invasion, and tube formation. Similarly, the in vivo Matrigel plug assay in C57BL/6 mice showed more pronounced reduction of blood vessels in the plugs containing 1 than those containing curcumin. Moreover, 1 suppressed tumor growth more effectively than curcumin in a U87MG mouse xenograft model by inhibiting angiogenesis. In vivo metabolite analysis by liquid chromatography/mass spectrometry demonstrated that 1 underwent markedly slower reductive metabolism than curcumin. Taken together, our results indicate that 1 has enhanced antiangiogenesis activity and suppression of tumor growth compared with curcumin, reflecting diminished reductive metabolism owing to the introduction of methyl groups at the C2 and C6 positions of curcumin.


