Methyl-6-gingerolCAS# 23513-10-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23513-10-2 | SDF | Download SDF |
| PubChem ID | 70697235 | Appearance | Oil |
| Formula | C18H28O4 | M.Wt | 308.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
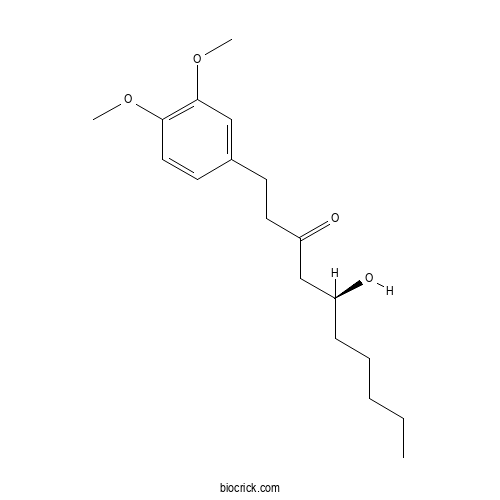
| Chemical Name | (5S)-1-(3,4-dimethoxyphenyl)-5-hydroxydecan-3-one | ||
| SMILES | CCCCCC(CC(=O)CCC1=CC(=C(C=C1)OC)OC)O | ||
| Standard InChIKey | CTGAPJBPSCUFRO-HNNXBMFYSA-N | ||
| Standard InChI | InChI=1S/C18H28O4/c1-4-5-6-7-15(19)13-16(20)10-8-14-9-11-17(21-2)18(12-14)22-3/h9,11-12,15,19H,4-8,10,13H2,1-3H3/t15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl-6-gingerol Dilution Calculator

Methyl-6-gingerol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2425 mL | 16.2127 mL | 32.4254 mL | 64.8508 mL | 81.0636 mL |
| 5 mM | 0.6485 mL | 3.2425 mL | 6.4851 mL | 12.9702 mL | 16.2127 mL |
| 10 mM | 0.3243 mL | 1.6213 mL | 3.2425 mL | 6.4851 mL | 8.1064 mL |
| 50 mM | 0.0649 mL | 0.3243 mL | 0.6485 mL | 1.297 mL | 1.6213 mL |
| 100 mM | 0.0324 mL | 0.1621 mL | 0.3243 mL | 0.6485 mL | 0.8106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 16-Acetoxy-7alpha-methoxyroyleanone
Catalog No.:BCX2084
CAS No.:109974-33-6
- Fischeroside A
Catalog No.:BCX2083
CAS No.:1307257-07-3
- 2-Hydroxy-6-methoxy-4-O-(6''-O-alpha-L-arabinofuranosyl-beta-D-glucopyranosyl)acetophenone
Catalog No.:BCX2082
CAS No.:1452160-87-0
- Isobiflorin
Catalog No.:BCX2081
CAS No.:152041-16-2
- Bracteanolide A
Catalog No.:BCX2080
CAS No.:1021432-39-2
- Graciliflorin E
Catalog No.:BCX2079
CAS No.:1413941-66-8
- 6,12,15-Trihydroxy-5,8,11,13-abietetra-7-one
Catalog No.:BCX2078
CAS No.:371155-09-8
- Gerardianin B
Catalog No.:BCX2077
CAS No.:2070919-73-0
- 16-Acetoxylsugiol
Catalog No.:BCX2076
CAS No.:1413941-65-7
- Aesculitannin B
Catalog No.:BCX2075
CAS No.:114612-77-0
- MTCA(1-Methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid)
Catalog No.:BCX2074
CAS No.:5470-37-1
- Strychnistenolide 6-O-acetate
Catalog No.:BCX2073
CAS No.:325969-56-0
- Rhamnetin 3-O-gentiobioside
Catalog No.:BCX2086
CAS No.:1786990-15-8
- Rhamnetin 3-sophoroside
Catalog No.:BCX2087
CAS No.:259234-17-8
- Suksdorfin
Catalog No.:BCX2088
CAS No.:53023-17-9
- Notoginsenoside SFt4
Catalog No.:BCX2089
CAS No.:1351360-30-9
- Albanin D
Catalog No.:BCX2090
CAS No.:134955-26-3
- 4'-O-Methylisoliquiritigenin
Catalog No.:BCX2091
CAS No.:476487-22-6
- Rutarin
Catalog No.:BCX2092
CAS No.:20320-81-4
- 5'-Methoxyoctahydrocurcumin
Catalog No.:BCX2093
CAS No.:718638-77-8
- 6-Hydroxykaempferol 7-glucoside
Catalog No.:BCX2094
CAS No.:70056-55-2
- Isosyringalide 3'-rhamnoside
Catalog No.:BCX2095
CAS No.:110326-99-3
- Tupichinol B
Catalog No.:BCX2096
CAS No.:497142-89-9
- Isorutarin
Catalog No.:BCX2097
CAS No.:53846-51-8
Putative mycobacterial efflux inhibitors from the seeds of Aframomum melegueta.[Pubmed:22789014]
J Nat Prod. 2012 Jul 27;75(7):1393-9.
In order to identify new putative efflux pump inhibitors that represent an appropriate target in antimycobacterial chemotherapy, nine paradol- and gingerol-related compounds (1-9) isolated from the seeds of Aframomum melegueta were assessed for their potential to inhibit ethidium bromide (EtBr) efflux in a Mycobacterium smegmatis model. Five of the compounds from A. melegueta and NMR spectroscopic data of the diketone 6-gingerdione (2) and its enolic tautomers, Methyl-6-gingerol (5) and rac-6-dihydroparadol (7), are presented herein for the first time. After determination of their antimycobacterial activities and modulatory effects on the MIC of antibiotics as well as their synergistic effects in combination with antibiotics against M. smegmatis mc(2) 155, their impact on EtBr accumulation and efflux was evaluated using a microtiter plate-based fluorometric assay. The compounds exhibited moderate to weak antimycobacterial activities, and the best modulators induced a 4- to 16-fold decrease of the MICs of EtBr and rifampicin as well as a reduction of the MIC of isoniazid with fractional inhibitory concentration index values indicating synergistic activities in some cases. 6-Paradol (3), 8-gingerol (6), and rac-6-dihydroparadol (7) were the most potent EtBr efflux inhibitors in M. smegmatis mc(2) 155, displaying EtBr efflux inhibiting activities comparable to reference inhibitors.


