Methenolone acetateCAS# 434-05-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 434-05-9 | SDF | Download SDF |
| PubChem ID | 252372 | Appearance | Powder |
| Formula | C22H32O3 | M.Wt | 344.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Metenolone acetate | ||
| Solubility | DMSO : ≥ 3.6 mg/mL (10.45 mM); | ||
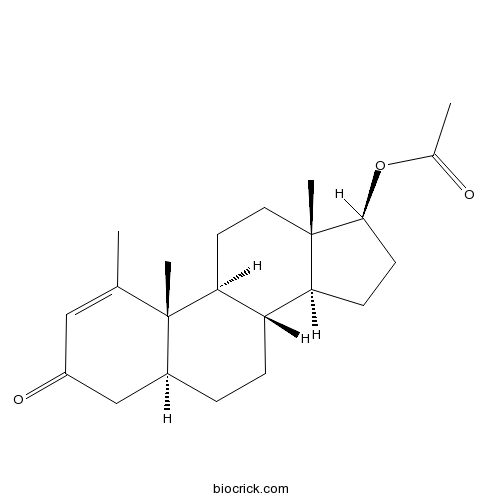
| Chemical Name | [(5S,8R,9S,10S,13S,14S,17S)-1,10,13-trimethyl-3-oxo-4,5,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl] acetate | ||
| SMILES | CC1=CC(=O)CC2C1(C3CCC4(C(C3CC2)CCC4OC(=O)C)C)C | ||
| Standard InChIKey | PGAUJQOPTMSERF-QWQRBHLCSA-N | ||
| Standard InChI | InChI=1S/C22H32O3/c1-13-11-16(24)12-15-5-6-17-18-7-8-20(25-14(2)23)21(18,3)10-9-19(17)22(13,15)4/h11,15,17-20H,5-10,12H2,1-4H3/t15-,17-,18-,19-,20-,21-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methenolone acetate Dilution Calculator

Methenolone acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9028 mL | 14.5138 mL | 29.0276 mL | 58.0552 mL | 72.5689 mL |
| 5 mM | 0.5806 mL | 2.9028 mL | 5.8055 mL | 11.611 mL | 14.5138 mL |
| 10 mM | 0.2903 mL | 1.4514 mL | 2.9028 mL | 5.8055 mL | 7.2569 mL |
| 50 mM | 0.0581 mL | 0.2903 mL | 0.5806 mL | 1.1611 mL | 1.4514 mL |
| 100 mM | 0.029 mL | 0.1451 mL | 0.2903 mL | 0.5806 mL | 0.7257 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Metenolone acetate is a long-acting anabolic steroid with weak androgenic properties. IC50 value: Target: Metenolone acetate is a naturally occurring compound, found within the adrenal glands of pregnant domesticated felines, and is supplied as the acetate ester for oral administration. Methenolone acetate can be suppressive of the hypothalamic-pituitary-gonadal axis. [1] Methenolone acetate is available as an injection or as an oral formulation. Methenolone is not 17-alpha-alkylated, but 1-methylated for oral bioavailability. This reduces the stress on the liver, but also the availability. [2]
References:
[1]. Metenolone enanthate
[2]. Metenolone
- Ethisterone
Catalog No.:BCC4478
CAS No.:434-03-7
- VU 0357121
Catalog No.:BCC4595
CAS No.:433967-28-3
- 3-O-Acetyloleanolic acid
Catalog No.:BCN5486
CAS No.:4339-72-4
- Toddaculine
Catalog No.:BCN3639
CAS No.:4335-12-0
- Boc-Tyr-OMe
Catalog No.:BCC3459
CAS No.:4326-36-7
- 6-Hydroxykaempferol
Catalog No.:BCN3334
CAS No.:4324-55-4
- Formoterol Hemifumarate
Catalog No.:BCC4349
CAS No.:43229-80-7
- Fenbendazole
Catalog No.:BCC1236
CAS No.:43210-67-9
- Skp2 Inhibitor C1
Catalog No.:BCC6298
CAS No.:432001-69-9
- 6-(5-Chloropyridin-2-yl)-7-hydroxy-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-one
Catalog No.:BCC8753
CAS No.:43200-81-3
- Zopiclone
Catalog No.:BCC9195
CAS No.:43200-80-2
- Allylestrenol
Catalog No.:BCC8814
CAS No.:432-60-0
- Oxymetholone
Catalog No.:BCC4692
CAS No.:434-07-1
- Lithocholic Acid
Catalog No.:BCC3805
CAS No.:434-13-9
- Nandrolone
Catalog No.:BCC9086
CAS No.:434-22-0
- Dacarbazine
Catalog No.:BCC1174
CAS No.:4342-03-4
- K 41498
Catalog No.:BCC5867
CAS No.:434938-41-7
- L-5-Hydroxytryptophan
Catalog No.:BCC8106
CAS No.:4350-09-8
- H-Arg(Tos)-OH
Catalog No.:BCC2867
CAS No.:4353-32-6
- 5-Hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one
Catalog No.:BCC8350
CAS No.:4354-76-1
- (-)-Curine
Catalog No.:BCN2673
CAS No.:436-05-5
- Diffractic Acid
Catalog No.:BCN8506
CAS No.:436-32-8
- Fangchinoline
Catalog No.:BCN5956
CAS No.:436-77-1
- Ajmaline
Catalog No.:BCN3867
CAS No.:4360-12-7
Development of an extraction method for anabolic androgenic steroids in dietary supplements and analysis by gas chromatography-mass spectrometry: Application for doping-control.[Pubmed:30118779]
Steroids. 2018 Oct;138:134-160.
Several studies have highlighted that nutritional supplements may contain undeclared anabolic steroids that are banned by the International Olympic Committee/World Anti-Doping Agency. Any kind of abuse with these drugs is extremely dangerous because of their side effects. Thus, the control of food additives in order to protect the best consumer health and to limit fraudulent practices in the field of sports is essential. This paper describes a simple and effective qualitative gas chromatography-mass spectrometry (GC-MS) method to detect anabolic androgenic steroids (AAS): androsterone, nandrolene, dehydroepiandrosterone, 5a-androstane-3beta, 17beta-diol, dihydrotestosterone, testosterone, Methenolone acetate, methandienone, boldenone and fluoxymesterone, in food supplements. Methyltestosterone was used as internal standard. Target compounds were extracted with a mixture of N-pentane and di-ethylether (7.5:2.5, v/v). A good extraction recovery was obtained by our method for all the AAS (R>88%). Crude extract was derivatized with N-methyl-N-trimethylsilyl-trifluoracetamide. Separation was performed on a GC connected to quadrupole MS detector using a 5% phenylmethylsiloxane fused silica capillary column (30mx0.25mm i.d.; film thickness, 0.25microm). Helium was used as carrier gas with a flow rate of 0.3microlmin(-1) (measured at 6.1 psi 190 degrees C). The MS was operated in electron ionization mode (70eV) and in selected ion monitoring (SIM). The mass spectra of the standard compounds were acquired in full SCAN mode (50-700m/z) by infusion of a reference solution at 50microg/ml. Three higher diagnostic ions were monitored for each compound of interest. All AAS get separated with good peak shapes and resolution factor. The total analysis time by our optimised method was only 20min. The developed method was validated according to Laboratories International Standard regulations for specificity, precision in both liquid and solid matrixes, and memory effect. The Tolerance Interval was judged true. The limit of detection was about 10ng/g for solid samples and 10ng/ml for liquid samples. The developed method was then applied to the research of steroids in nine Tunisian commercially dietary supplements using for each compound of interest SIM mode for screening then SCAN mode for confirmation. One of the monitoring samples was positive to methandienone not declared on the label. Our analytical method can be beneficial for AAS screening in dietary supplements.
Chronic anabolic androgenic steroid usage associated with acute coronary syndrome in bodybuilder.[Pubmed:27239638]
Turk J Emerg Med. 2016 Mar 10;16(1):35-7.
INTRODUCTION: It has been argued in current studies that anabolic androgenic steroids (AAS) are misused by a great number of bodybuilders and athletes. However, there is diverse and often conflicting scientific data on the cardiac and metabolic complications caused by the misuse of AAS. There may be various reasons for myocardial infarction (MI) with normal coronary arteries. However, for the majority of patients, the exact cause is still unknown. CASE REPORT: A 32 year-old male who was complaining about severe chest pain was admitted to our emergency department. He had been taking Methenolone acetate 200 mg weekly for a period of three years for body building. His cardiac markers were significantly elevated and electrocardiogram (ECG) showed peaked T waves in all derivations, which did not show ST elevation or depression. Both right and left coronary artery systems were found to be completely normal as a result of the angiogram. CONCLUSION: The purpose of this study is to show that AAS induced MI can be encountered with normal coronary arteries during acute coronary syndrome.
Myelodysplastic syndrome treated effectively with testosterone enanthate.[Pubmed:21449972]
Int J Urol. 2011 Jun;18(6):469-71.
We report a case of myelodysplastic syndrome (MDS) treated effectively with testosterone enanthate. A 70-year-old man was diagnosed with low-risk MDS in 1998, and he was first given Methenolone acetate orally because of gradual progression of anemia and thrombocytopenia. However, this treatment was not effective, so we changed the treatment to testosterone enanthate because of his symptoms with late-onset hypogonadism. Three months after testosterone replacement therapy (TRT), anemia and thrombocytopenia had improved, and mean platelet count and hemoglobin had significant increases from 2.36 +/- 0.45 x 10(4) to 3.83 +/- 0.78 x 10(4) /microL, and from 11.7 +/- 0.81 to 15.2 +/- 1.00 g/dL, respectively, which contributed to a decrease in platelet transfusion requirement. Since then, the patient has been on a good clinical course. The present case suggests that testosterone enanthate administration could be an alternative treatment for men with MDS, even in the case where treatment with anabolic-androgenic steroids is not successful, and suggests another interesting effect of TRT on platelets.
In vitro metabolic studies using homogenized horse liver in place of horse liver microsomes.[Pubmed:21381223]
Drug Test Anal. 2011 Jun;3(6):393-9.
The study of the metabolism of drugs, in particular steroids, by both in vitro and in vivo methods has been carried out in the authors' laboratory for many years. For in vitro metabolic studies, the microsomal fraction isolated from horse liver is often used. However, the process of isolating liver microsomes is cumbersome and tedious. In addition, centrifugation at high speeds (over 100 000 g) may lead to loss of enzymes involved in phase I metabolism, which may account for the difference often observed between in vivo and in vitro results. We have therefore investigated the feasibility of using homogenized horse liver instead of liver microsomes with the aim of saving preparation time and improving the correlation between in vitro and in vivo results. Indeed, the preparation of the homogenized horse liver was very simple, needing only to homogenize the required amount of liver. Even though no further purification steps were performed before the homogenized liver was used, the cleanliness of the extracts obtained, based on gas chromatography-mass spectrometry (GC-MS) analysis, was similar to that for liver microsomes. Herein, the results of the in vitro experiments carried out using homogenized horse liver for five anabolic steroids-turinabol, Methenolone acetate, androst-4-ene-3,6,17-trione, testosterone, and epitestosterone-are discussed. In addition to the previously reported in vitro metabolites, some additional known in vivo metabolites in the equine could also be detected. As far as we know, this is the first report of the successful use of homogenized liver in the horse for carrying out in vitro metabolism experiments. Copyright (c) 2011 John Wiley & Sons, Ltd.
Screening of anabolic steroids in horse urine by liquid chromatography-tandem mass spectrometry.[Pubmed:15862683]
J Pharm Biomed Anal. 2005 Apr 29;37(5):1031-8.
Anabolic steroids have the capability of improving athletic performance and are banned substances in the Olympic games as well as in horseracing and equestrian competitions. The control of their abuse in racehorses is traditionally performed by detecting the presence of anabolic steroids and/or their metabolite(s) in urine samples using gas chromatography-mass spectrometry (GC-MS). However, this approach usually requires tedious sample processing and chemical derivatisation steps and could be very insensitive in detecting certain steroids. This paper describes a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS-MS) method for the detection of anabolic steroids that are poorly covered by GC-MS. Enzyme-treated urine was processed by solid-phase extraction (SPE) using a Bond Elut Certify cartridge, followed by a base wash for further cleanup. Separation of the steroids was carried out on a reversed-phase DB-8 column using 0.1% acetic acid and methanol as the mobile phase in a gradient elution programme. The mass spectrometer for the detection of the steroids was operated in the positive electrospray ionisation (ESI) mode with multiple reaction monitoring (MRM). Urine samples fortified with 15 anabolic steroids (namely, androstadienone, 1-androstenedione, bolasterone, boldione, 4-estrenedione, gestrinone, methandrostenolone, methenolone, 17alpha-methyltestosterone, norbolethone, normethandrolone, oxandrolone, stenbolone, trenbolone and turinabol) at low ng/mL levels were consistently detected. No significant matrix interference was observed at the retention times of the targeted ion masses in blank urine samples. The method specificity, sensitivity, precision, recoveries, and the performance of the enzyme hydrolysis step were evaluated. The successful application of the method to analyse Methenolone acetate administration urine samples demonstrated that the method could be effective in detecting anabolic steroids and their metabolites in horse urine.
Measurement of 19-nortestosterone and its esters in equine plasma by high-performance liquid chromatography with tandem mass spectrometry.[Pubmed:11006593]
Rapid Commun Mass Spectrom. 2000;14(19):1835-40.
A high-performance liquid chromatographic-tandem mass spectrometric (HPLC/MS/MS) method for the determination of 19-nortestosterone and its esters (cyclopentanepropionate, phenylpropionate, and decanoate) in equine plasma is achieved using an atmospheric pressure chemical ionization (APCI) interface in selected reaction monitoring (SRM) mode. The two internal standards used were 16,16, 17-(2)H(3)-19-nortestosterone for 19-nortestosterone and Methenolone acetate for its esters. The steroids studied were extracted from plasma samples with a mixture of diethyl ether/n-hexane (9:1, v/v). The quantification limits for 19-nortestosterone, 19-nortestosterone cyclopentanepropionate, 19-nortestosterone phenylpropionate, and 19-nortestosterone decanoate were 0.16, 5.0, 0.1, and 2.0 ng/mL, respectively, when 2 mL of plasma were used. The recoveries of most of the steroids were 71.6-101.0% except for the decanoate, which could be recovered to about 39.8%. The responses were linear, with correlation coefficients varying from 0.9897 to 0.9999 in the concentration range of 0.1 to 50.0 ng/mL for the steroids studied. When applied to equine (mare) plasma samples, the present method allowed detection of 19-nortestosterone up to 23 days after an intra-muscular injection of 400 mg as the decanoate.
[Hemophagocytic syndrome due to miliary tuberculosis in the course of aplastic anemia].[Pubmed:9637891]
Rinsho Ketsueki. 1998 May;39(5):392-7.
We report a 63 year-old female with aplastic anemia (AA) who was complicated with hemophagocytic syndrome induced by systemic miliary tuberculosis. Two years before admission to our hospital, she was diagnosed as AA and had been treated with granulocyte colony-stimulating factor, erythropoietin and Methenolone acetate. In May, 1996, She was transferred to our hospital because of high fever and exacervation of pancytopenia. She showed severe pancytopenia, and an increase in macrophages showing remarkable erythrophagocytosis and decrease in hemopoietic cells in the bone marrow. In initial examination, high titer of IgM antibody to herpes simplex virus type I was identified and methylprednisolone pulse therapy was started under the diagnosis of virus associated hemophagocytic syndrome. Ten days later, however, she died for intestinal hemorrhage followed by multiorgan failure. In autopsy, multiple epitheloid cell granulomas with acid-fast bacilli were found in bone marrow, lungs, liver, spleen and kidneys.


