Malabaricone CCAS# 63335-25-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63335-25-1 | SDF | Download SDF |
| PubChem ID | 100313 | Appearance | Powder |
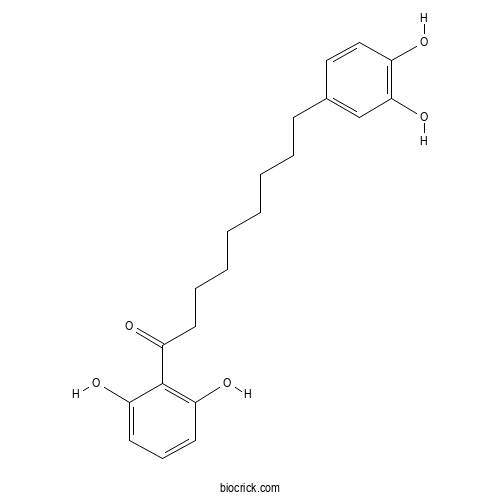
| Formula | C21H26O5 | M.Wt | 358.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2,6-dihydroxyphenyl)-9-(3,4-dihydroxyphenyl)nonan-1-one | ||
| SMILES | C1=CC(=C(C(=C1)O)C(=O)CCCCCCCCC2=CC(=C(C=C2)O)O)O | ||
| Standard InChIKey | HCOZRFYGIFMIEX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Malabaricone C Dilution Calculator

Malabaricone C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7902 mL | 13.9509 mL | 27.9018 mL | 55.8036 mL | 69.7545 mL |
| 5 mM | 0.558 mL | 2.7902 mL | 5.5804 mL | 11.1607 mL | 13.9509 mL |
| 10 mM | 0.279 mL | 1.3951 mL | 2.7902 mL | 5.5804 mL | 6.9754 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.558 mL | 1.1161 mL | 1.3951 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.558 mL | 0.6975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isosecotanapartholide
Catalog No.:BCN0839
CAS No.:102926-01-2
- 8-Epiloganin
Catalog No.:BCN0838
CAS No.:79172-04-6
- Otobaphenol
Catalog No.:BCN0837
CAS No.:10240-16-1
- Rhamnocitrin 3-galactoside
Catalog No.:BCN0836
CAS No.:99878-05-4
- Ganolucidic acid D
Catalog No.:BCN0835
CAS No.:102607-22-7
- 2,6-Dihydroxyacetophenone-4-O-[4',6'-(S)-hexahydroxydiphenoyl]-beta-D-glucose
Catalog No.:BCN0834
CAS No.:1781226-44-5
- Ganoderenic acid K
Catalog No.:BCN0833
CAS No.:942950-94-9
- Gardoside methyl ester
Catalog No.:BCN0832
CAS No.:93930-20-2
- Oxytroflavoside A
Catalog No.:BCN0831
CAS No.:1391144-80-1
- 12beta-Acetoxy-3beta-hydroxy-7,11,15,23-tetraoxo-lanost-8,20-diene-26-oic acid
Catalog No.:BCN0830
CAS No.:1085338-75-5
- Oxytroflavoside E
Catalog No.:BCN0829
CAS No.:1391144-84-5
- Pinocembrin 7-O-(4'',6''-hexahydroxydiphenoyl)-beta-D-glucose
Catalog No.:BCN0828
CAS No.:1825287-22-6
- Baishouwubenzophenone
Catalog No.:BCN0841
CAS No.:115834-34-9
- Malabaricone A
Catalog No.:BCN0842
CAS No.:63335-23-9
- Silybin A
Catalog No.:BCN0843
CAS No.:36804-17-8
- 4-p-Menthan-1,8-diol
Catalog No.:BCN0844
CAS No.:565-48-0
- Neomogroside
Catalog No.:BCN0845
CAS No.:189307-15-1
- Mogroside I-E1
Catalog No.:BCN0846
CAS No.:88901-39-7
- Mogroside I-A1
Catalog No.:BCN0847
CAS No.:88901-46-6
- Mogroside II-B
Catalog No.:BCN0848
CAS No.:942615-25-0
- 11-Oxomogroside I
Catalog No.:BCN0849
CAS No.:918972-06-2
- 11-Deoxymogroside IIIE
Catalog No.:BCN0850
CAS No.:1793003-47-0
- Siraitic acid B
Catalog No.:BCN0851
CAS No.:183374-16-5
- Siraitic acid A
Catalog No.:BCN0852
CAS No.:183374-15-4
Giganteone A and malabaricone C as potential pharmacotherapy for diabetes mellitus.[Pubmed:33593208]
Nat Prod Res. 2022 Mar;36(6):1581-1586.
The use of antidiabetic agents which control glycemic levels in the blood and simultaneously inhibit oxidative stress is an important strategy in the prevention of Diabetes Mellitus and its complications. In our previous study, Malabaricone C (3) and its dimer, giganteone A (5) exhibited significant DPPH free radical scavenging activities which were lower than the activity of the positive control, ascorbic acid. These compounds were evaluated for their alpha-glucosidase inhibitory activities at different concentrations (0.02-2.5 mM) in the present study. Compounds 3 (IC50 59.61 microM) and 5 (IC50 39.52 microM) were identified as active alpha-glucosidase inhibitors, each respectively being 24 and 37 folds more potent than the standard inhibitor, acarbose. Based on the molecular docking studies, compounds 3 and 5 docked into the active site of the alpha-glucosidase enzyme, forming mainly hydrogen bonds in the active site.
Phytochemical and pharmacological properties of Myristica fragrans Houtt.: an updated review.[Pubmed:33206347]
Arch Pharm Res. 2020 Nov;43(11):1067-1092.
Myristica fragrans Houtt. (Myristicaceae), an aromatic evergreen tree, is well known as a commercial source of mace (aril) and nutmeg (seed), which have long been widely used as spices in the culinary field. In addition, various parts of M. fragrans have been used in folk medicine for treating several diseases. Since its extensive uses in the culinary sector and folk medicine, M. fragrans has long attracted a great deal of attention from pharmacologists and chemists. Numerous studies have indicated that M. fragrans contains diverse phytochemicals such as lignans, neolignans, diphenylalkanes, phenylpropanoids, and terpenoids, which exhibit many of pharmacological activities. Among them, macelignan (1), meso-dihydroguaiaretic acid (2), myristicin (111), and Malabaricone C (Mal C, 104) are the most active compounds. The aim of this review is to comprehensively summarize the phytochemical and pharmacological properties of M. fragrans that have reported to date.
Evaluation of Antioxidant and Anti-alpha-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans.[Pubmed:33171671]
Molecules. 2020 Nov 8;25(21). pii: molecules25215198.
Myristica fragrans is a well-known species for flavoring many food products and for formulation of perfume and medicated balm. It is also used to treat indigestion, stomach ulcers, liver disorders, and, as emmenagogue, diaphoretic, diuretic, nervine, and aphrodisiac. We examined antioxidant properties and bioactive compounds in various solvent extracts from the seeds of M. fragrans. Methanol, ethanol, and acetone extracts exhibited relatively strong antioxidant activities by 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), superoxide radical, and hydroxyl radical scavenging tests. Furthermore, methanol extracts also displayed significant anti-alpha-glucosidase activity. Examined and compared to the various solvent extracts for their chemical compositions using HPLC analysis, we isolated the ten higher content compounds and analyzed antioxidant and anti-alpha-glucosidase activities. Among the isolates, dehydrodiisoeugenol, malabaricone B and Malabaricone C were main antioxidant components in seeds of M. fragrans. Malabaricone C exhibited stronger antioxidant capacities than others based on lower half inhibitory concentration (IC50) values in DPPH and ABTS radical scavenging assays, and it also showed significant inhibition of alpha-glucosidase. These results shown that methanol was found to be the most efficient solvent for extracting the active components from the seeds of M. fragrans, and this material is a potential good source of natural antioxidant and alpha-glucosidase inhibitor.
Thiol antioxidants sensitize malabaricone C induced cancer cell death via reprogramming redox sensitive p53 and NF-kappaB proteins in vitro and in vivo.[Pubmed:31945496]
Free Radic Biol Med. 2020 Feb 20;148:182-199.
Specific focus on "redox cancer therapy" by targeting drugs to redox homeostasis of the cancer cells is growing rapidly. Recent clinical studies showed that N-acetyl cysteine (NAC) treatment significantly decreased the metabolic heterogeneity and reduced Ki67 (a proliferation marker) with simultaneous enhancement in apoptosis of tumor cells in patients. However, it is not yet precisely known how thiol antioxidants enhance killing of cancer cells in a context dependent manner. To this end, we showed that a dietary compound, Malabaricone C (mal C) generated copious amounts of reactive oxygen species (ROS) and also reduced GSH level in lung cancer cells. Paradoxically, although antioxidants supplementation reduced mal C-induced ROS, thiol-antioxidants (NAC/GSH) restored intracellular GSH level but enhanced DNA DSBs and apoptotic cell death induced by mal C. Our results unraveled two tightly coupled biochemical mechanisms attributing this sensitization process by thiol antioxidants. Firstly, thiol antioxidants enable the "catechol-quinone redox cycle" of mal C and ameliorate ROS generation and bio-molecular damage (DNA and protein). Secondly, thiol antioxidants cause rapid glutathionylation of transcription factors [p53, p65 (NF-kappaB) etc.], oxidized by mal C, and abrogates their nuclear sequestration and transcription of the anti-apoptotic genes. Furthermore, analyses of the mitochondrial fractions of p53 expressing and silenced cells revealed that cytoplasmic accumulation of glutathionylated p53 (p53-SSG) triggers a robust mitochondrial death process. Interestingly, mutation of redox sensitive cysteine residues at 124, 141 and 182 position in p53 significantly reduces mal C plus NAC mediated sensitization of cancer cells. The preclinical results, in two different tumor models in mice, provides further support our conclusion that NAC is able to sensitize mal C induced suppression of tumor growth in vivo.
Malabaricone C Attenuates Nonsteroidal Anti-Inflammatory Drug-Induced Gastric Ulceration by Decreasing Oxidative/Nitrative Stress and Inflammation and Promoting Angiogenic Autohealing.[Pubmed:31830804]
Antioxid Redox Signal. 2020 Apr 10;32(11):766-784.
Aims: Nonsteroidal anti-inflammatory drugs (NSAIDs), among the most commonly used drugs worldwide, are associated with gastrointestinal (GI) complications that severely limit the clinical utility of this essential class of pain medications. Here, we mechanistically dissect the protective impact of a natural product, Malabaricone C (Mal C), on NSAID-induced gastropathy. Results: Mal C dose dependently diminished erosion of the stomach lining and inflammation in mice treated with NSAIDs with the protective impact translating to improvement in survival. By decreasing oxidative and nitrative stress, Mal C treatment prevented NSAID-induced mitochondrial dysfunction and cell death; nuclear factor kappa-light-chain enhancer of activated B cell induction, release of proinflammatory cytokines and neutrophil infiltration; and disruptions in the vascular endothelial growth factor/endostatin balance that contributes to mucosal autohealing. Importantly, Mal C failed to impact the therapeutic anti-inflammatory properties of multiple NSAIDs in a model of acute inflammation. In all assays tested, Mal C proved as or more efficacious than the current first-line therapy for NSAID-dependent GI complications, the proton pump inhibitor omeprazole. Innovation: Given that omeprazole-mediated prophylaxis is, itself, associated with a shift in NSAID-driven GI complications from the upper GI to the lower GI system, there is a clear and present need for novel therapeutics aimed at ameliorating NSAID-induced gastropathy. Mal C provided significant protection against NSAID-induced gastric ulcerations impacting multiple critical signaling cascades contributing to inflammation, cell loss, extracellular matrix degradation, and angiogenic autohealing. Conclusion: Thus, Mal C represents a viable lead compound for the development of novel gastroprotective agents.
Phenolic compounds from nutmeg (Myristica fragrans Houtt.) inhibit the endocannabinoid-modulating enzyme fatty acid amide hydrolase.[Pubmed:31595522]
J Pharm Pharmacol. 2019 Dec;71(12):1879-1889.
OBJECTIVES: The study aimed to identify nutmeg compounds that indirectly interact with the endocannabinoid system through inhibition of the fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) enzymes. METHODS: Thirteen compounds were screened for FAAH and MAGL inhibition. Compounds demonstrating significant FAAH inhibition were evaluated to determine the halfmaximal inhibitory concentration (IC50 ). The most potent compound was investigated in the elevated plus maze (EPM) rodent anxiety model. KEY FINDINGS: Three compounds, licarin A (9), 5'-methoxylicarin A (8) and Malabaricone C (6) were most active in inhibiting FAAH with IC50 of 7.02 mum +/- 2.02, 4.57 mum +/- 0.66 and 38.29 mum +/- 6.18, respectively. None of the purified compounds showed significant MAGL inhibition. Because of its relative high potency and selectivity, compound 8 was further evaluated in the EPM animal model of anxiety. The compound showed significant increase in number of open arm entries (P < 0.05) when administered at 120 mg/kg dose. No effect was observed on the locomotor activity. CONCLUSIONS: Results collected introduce active nutmeg compounds as potential leads for further development. Of the three compounds, 8 possesses highest potency and FAAH selectivity as well as anxiolytic activity. Furthermore, in vivo testing in appropriate behavioural animal paradigms is warranted.
Malabaricone C as Natural Sphingomyelin Synthase Inhibitor against Diet-Induced Obesity and Its Lipid Metabolism in Mice.[Pubmed:31413799]
ACS Med Chem Lett. 2019 Jul 3;10(8):1154-1158.
The interaction between natural occurring inhibitors and targeted membrane proteins could be an alternative medicinal strategy for the treatment of metabolic syndrome, notably, obesity. In this study, we identified malabaricones A-C and E (1-4) isolated from the fruits of Myristica cinnamomea King as natural inhibitors for sphingomyelin synthase (SMS), a membrane protein responsible for sphingolipid biosynthesis. Having the most promising inhibition, oral administration of compound 3 exhibited multiple efficacies in reducing weight gain, improving glucose tolerance, and reducing hepatic steatosis in high fat diet-induced obesity mice models. Liver lipid analysis revealed a crucial link between the SMS activities of compound 3 and its lipid metabolism in vitro and in vivo. The nontoxic nature of compound 3 makes it a suitable candidate in search of drugs which can be employed in the treatment and prevention of obesity.
Antidiabetic potential of phytochemicals isolated from the stem bark of Myristica fatua Houtt. var. magnifica (Bedd.) Sinclair.[Pubmed:29789207]
Bioorg Med Chem. 2018 Jul 23;26(12):3461-3467.
Phytochemical investigation of the stem bark of Myristica fatua Houtt. led to the isolation of a new compound 1 (3-tridecanoylbenzoic acid), along with six known acylphenols (2-7). All the compounds displayed moderate inhibitory activity on alpha-amylase and significant activity on alpha-glucosidase; however malabaricone B (6) and C (7) were identified as potent alpha-glucosidase inhibitors with IC50 values of 63.70+/-0.546, and 43.61+/-0.620microM respectively. Acylphenols (compounds 3-7) also showed significant antiglycation property. The molecular docking and dynamics simulation studies confirmed the efficient binding of Malabaricone C with C-terminus of human maltase-glucoamylase (2QMJ). Malabaricone B also enhanced the 2-NBDG [2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxy glucose] uptake in L6 myotubes. These findings demonstrate that acylphenols isolated from Myristica fatua Houtt. can be considered as a lead scaffold for the treatment of type II diabetes mellitus.
Degranulation inhibitors from the arils of Myristica fragrans in antigen-stimulated rat basophilic leukemia cells.[Pubmed:29336005]
J Nat Med. 2018 Mar;72(2):464-473.
A methanol extract of mace, the aril of Myristica fragrans (Myristicaceae), was found to inhibit the release of beta-hexosaminidase, a marker of antigen-IgE-stimulated degranulation in rat basophilic leukemia cells (RBL-2H3, IC50 = 45.7 mug/ml). From the extract, three new 8-O-4' type neolignans, maceneolignans I-K (1-3), were isolated, and the stereostructures of 1-3 were elucidated based on spectroscopic and chemical evidence. Among the isolates, maceneolignans A (5), D (6), and H (8), (-)-(8R)-(8')-4-hydroxy-3,3',5'-trimethoxy-8-O-4'-neolignan (13), (-)-(8R)-(8')-3,4,5,3',5'-pentamethoxy-8-O-4'-neolignan (14), (-)-erythro-(7R,8S)-(8')-7-acetoxy-3,4-methylenedioxy-3',5'-dimethoxy-8-O-4'-neol ignan (17), (+)-licarin A (20), nectandrin B (24), verrucosin (25), and Malabaricone C (29) were investigated as possible degranulation inhibitors (IC50 = 20.7-63.7 muM). These inhibitory activities were more potent than those of the antiallergic agents tranilast (282 muM) and ketotifen fumalate (158 muM). Compounds 5, 25, and 29 also inhibited antigen-stimulated tumor necrosis factor-alpha production (IC50 = 39.5-51.2 muM), an important process in the late phase of type I allergic reactions.
Total Syntheses of Malabaricones B and C via a Cross-Metathesis Strategy.[Pubmed:28581739]
J Nat Prod. 2017 Jun 23;80(6):1776-1782.
The malabaricones A-D belong to the class of diarylnonanoids isolated from the Myristicaceae family of plants. Although Malabaricone C displayed various interesting biological activities, its isolation remains tedious due to its close chemical similarity to malabaricones A, B, and D. Therefore, development of an efficient synthesis route has become essential to cater to the need of large amounts of Malabaricone C for its pharmacological profiling. So far there is only one report of the synthesis of Malabaricone C through a lengthy sequence of reactions. We have developed an efficient and short route for the syntheses of malabaricones B and C, which will also provide a convenient access to all other members of the malabaricone family. Synthesis of an important building block, omega-aryl heptyl bromide, employed in the synthesis was realized by adopting a cross-metathesis reaction as the key step.
Sialidase inhibitory activity of diarylnonanoid and neolignan compounds extracted from the seeds of Myristica fragrans.[Pubmed:28551100]
Bioorg Med Chem Lett. 2017 Jul 15;27(14):3060-3064.
Sialidases are key virulence factors that remove sialic acid from host cell surface glycans, thus unmasking receptors to facilitate bacterial adherence and colonization. In this study, we report the isolation and characterization of novel inhibitors of the Streptococcus pneumoniae sialidases NanA, NanB, and NanC from Myristica fragrans seeds. Of the isolated compounds (1-12), Malabaricone C showed the most pneumococcal sialidases inhibition (IC50 of 0.3muM for NanA, 3.6muM for NanB, and 2.9muM for NanC). These results suggested that Malabaricone C and neolignans could be potential agents for combating S. pneumoniae infection agents.
HPLC-Guided Isolation, Purification and Characterization of Phenylpropanoid and Phenolic Constituents of Nutmeg Kernel (Myristica fragrans).[Pubmed:27396199]
Nat Prod Commun. 2016 Apr;11(4):483-8.
Many studies on the biological activities of nutmeg continue to appear in the literature. The most common targets include GIT, CNS, oxidative stress and inflammation. However, results obtained from most studies are often inconsistent due to the variability of utilized samples, lack of standardized nutmeg products or insufficient amounts of pure compounds for comprehensive follow-up investigation. To address the consistency and supply issue we utilized available technology to develop a reproducible procedure for preparation of specific extracts and isolation of the major phenolic constituents present in nutmeg kemel. A well-defined and reproducible sequence of extraction, fractionation and chromatographic purification was adopted and was guided by HPLC fingerprinting. Spectroscopic methods, mainly NMR, and mass spectrometry were utilized to identify each compound. Thirteen compounds were isolated in a pure form and identified as: elemicin (1), isoelemicin (2), myristicin (4), surinamensin (5), Malabaricone C (6), 2-(3'-allyl-2',6'-dimethoxy-phenyloxy)-l- acetoxy-(3,4-dimethoxyphenyl)-propyl ester (7), methoxylicarin A (8), licarin A (9), malabaricone B (10), licarin C (11), 5'-methoxylicarin B (12), licarin B (13), and 2-(3'-allyl-2',6'-dimethoxy-phenyloxy)-l-methyl-5-methoxy-1,2-dihydrobenzofuran (3, a new compound). With repeated isolation runs, these pure compounds can be prepared in quantities sufficient for biological evaluation as needed. The availability of purified compounds will also allow the development of specific, accurate, and sensitive analytical procedures for pharmacokinetic studies and for quality control of nutmeg products. Both aspects are essential for nutmeg-focused drug discovery. The same approach can also be adapted to other medicinal plants of potential interest.
Mechanism of the anti-hypertensive property of the naturally occurring phenolic, malabaricone C in DOCA-salt rats.[Pubmed:26503350]
Free Radic Res. 2016;50(1):111-21.
In this study, we studied whether chronic oral administration of the natural antioxidant, Malabaricone C (mal C) can reduce blood pressure (BP) and attenuate cardio-vascular remodeling in deoxycorticosterone acetate (DOCA)-salt hypertensive rats. The dose of mal C for its anti-hypertensive action was optimized by measuring the systolic BP (SBP). DOCA-salt rats showed very high SBP, associated with organ hypertrophy, collagen depositions, and inflammatory infiltrations in cardiac and aortic sections, reduced plasma total antioxidant status and NO level, and increased levels of TBARS, PGI2 as well as vasoconstrictors (AVP, Big ET, and ET-1). DOCA-salt also reduced smooth muscle- and endothelium-dependent vascular relaxation in rats. Mal C reversed all these changes of the DOCA-salt rats and improved their vascular reactivity. Mal C exerts anti-hypertensive property in DOCA-salt rats by reducing oxidative stress and organ hypertrophy, and improving endothelial and vascular functions. Given that mal C has appreciable natural abundance and is non-toxic to rodents, further studies would help in establishing its medicinal potential against hypertension.


