MacluraxanthoneCAS# 5848-14-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

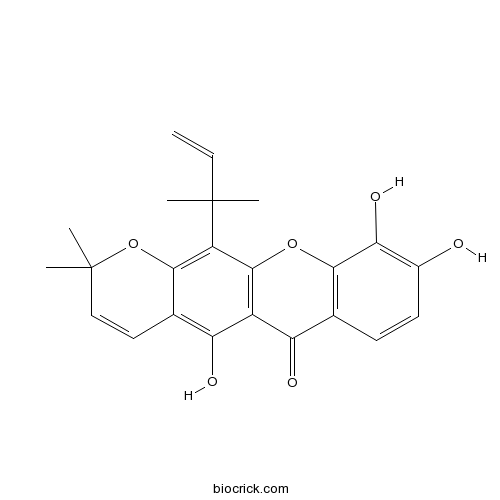
| Cas No. | 5848-14-6 | SDF | Download SDF |
| PubChem ID | 5281646 | Appearance | Yellow powder |
| Formula | C23H22O6 | M.Wt | 394.4 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,9,10-trihydroxy-2,2-dimethyl-12-(2-methylbut-3-en-2-yl)pyrano[3,2-b]xanthen-6-one | ||
| SMILES | CC1(C=CC2=C(C3=C(C(=C2O1)C(C)(C)C=C)OC4=C(C3=O)C=CC(=C4O)O)O)C | ||
| Standard InChIKey | XRVLGJCHUWXTDX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H22O6/c1-6-22(2,3)15-19-12(9-10-23(4,5)29-19)17(26)14-16(25)11-7-8-13(24)18(27)20(11)28-21(14)15/h6-10,24,26-27H,1H2,2-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Macluraxanthone Dilution Calculator

Macluraxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5355 mL | 12.6775 mL | 25.355 mL | 50.7099 mL | 63.3874 mL |
| 5 mM | 0.5071 mL | 2.5355 mL | 5.071 mL | 10.142 mL | 12.6775 mL |
| 10 mM | 0.2535 mL | 1.2677 mL | 2.5355 mL | 5.071 mL | 6.3387 mL |
| 50 mM | 0.0507 mL | 0.2535 mL | 0.5071 mL | 1.0142 mL | 1.2677 mL |
| 100 mM | 0.0254 mL | 0.1268 mL | 0.2535 mL | 0.5071 mL | 0.6339 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Deoxymikanolide
Catalog No.:BCN9420
CAS No.:23753-57-3
- 4,5-Dihydropiperlonguminine
Catalog No.:BCN9419
CAS No.:23512-53-0
- Dihydromikanolide
Catalog No.:BCN9418
CAS No.:23758-04-5
- 2,6-Dihydroxyxanthone
Catalog No.:BCN9417
CAS No.:838-11-9
- Minisecolide C
Catalog No.:BCN9416
CAS No.:1967030-77-8
- Withaphysalin A
Catalog No.:BCN9415
CAS No.:57423-72-0
- Withaphysalin E
Catalog No.:BCN9414
CAS No.:118985-24-3
- Withaphysalin S
Catalog No.:BCN9413
CAS No.:949172-13-8
- Methyl (9Z,11E)-13-hydroxyoctadeca-9,11-dienoate
Catalog No.:BCN9412
CAS No.:109837-85-6
- Physaminimin C
Catalog No.:BCN9411
CAS No.:1582259-03-7
- Physaminimin D
Catalog No.:BCN9410
CAS No.:1582259-05-9
- Hyperxanthone
Catalog No.:BCN9409
CAS No.:99481-41-1
- 4-Hydroxyxanthone
Catalog No.:BCN9422
CAS No.:14686-63-6
- 3-(Hydroxyacetyl)indole
Catalog No.:BCN9423
CAS No.:2400-51-3
- Minisecolide D
Catalog No.:BCN9424
CAS No.:1967030-78-9
- Lipodeoxyaconitine
Catalog No.:BCN9425
CAS No.:244190-83-8
- Lipoaconitine
Catalog No.:BCN9426
CAS No.:81941-14-2
- 3-epi-Dihydroscandenolide
Catalog No.:BCN9427
CAS No.:1137951-08-6
- cis-Shegansu B
Catalog No.:BCN9428
CAS No.:865474-99-3
- Methyl 2,4,6-trihydroxybenzoate
Catalog No.:BCN9429
CAS No.:3147-39-5
- Elatoside E
Catalog No.:BCN9430
CAS No.:156980-30-2
- 1H-Indole-3-carbaldehyde
Catalog No.:BCN9431
CAS No.:487-89-8
- Scandenolide
Catalog No.:BCN9432
CAS No.:23758-16-9
- Neoorthosiphol A
Catalog No.:BCN9433
CAS No.:243448-72-8
Immuno-modulatory effects of macluraxanthone on macrophage phenotype and function.[Pubmed:32508145]
Nat Prod Res. 2020 Jun 8:1-6.
Macluraxanthone was previously reported to have many biological activities, including anti-cholinesterase, anti-oxidant, anti-cancer, anti-malarial and anti-inflammatory effects. The aim of the current study was to further characterise the effect of Macluraxanthone on human macrophage, a type of immune cell that has been implicated in the development of various inflammatory diseases. The expression of surface markers and cytokine production by THP-1 human macrophages following treatment with Macluraxanthone were investigated. Macluraxanthone was shown to promote polarisation of M1-like pro-inflammatory macrophages by increasing the percentage of macrophages expressing CD86, while decreasing their CD14, CD11b and CD80 expression. However, in the presence of the pro-inflammatory stimulus lipopolysaccharide, Macluraxanthone significantly decreased TNF-alpha and IL-10 cytokine production.
Gerontoxanthone I and Macluraxanthone Induce Mitophagy and Attenuate Ischemia/Reperfusion Injury.[Pubmed:32351391]
Front Pharmacol. 2020 Apr 15;11:452.
Mitophagy is a crucial process in controlling mitochondrial biogenesis. Balancing mitophagy and mitochondrial functions is required for maintaining cellular homeostasis. In this study, we found that Gerontoxanthone I (GeX1) and Macluraxanthone (McX), xanthone derivatives isolated from Garcinia bracteata C. Y. Wu ex Y. H. Li, induced Parkin puncta accumulation and promoted mitophagy. GeX1 and McX treatment induced the degradation of mitophagy-related proteins such as Tom20 and Tim23. GeX1 and McX directly stabilized PTEN-induced putative kinase 1 (PINK1) on the outer membrane of the mitochondria, and then recruited Parkin to mitochondria. This significantly induced phosphorylation and ubiquitination of Parkin, suggesting that GeX1 and McX mediate mitophagy through the PINK1-Parkin pathway. Transfecting ParkinS65A or pretreated MG132 abolished the induction effects of GeX1 and McX on mitophagy. Furthermore, GeX1 and McX treatment decreased cell death and the level of ROS in an ischemia/reperfusion (IR) injury model in H9c2 cells compared to a control group. Taken together, our data suggested that GeX1 and McX induce PINK1-Parkin-mediated mitophagy and attenuate myocardial IR injury in vitro.
Anti-HIV and cytotoxic biphenyls, benzophenones and xanthones from stems, leaves and twigs of Garcinia speciosa.[Pubmed:29304383]
Phytochemistry. 2018 Mar;147:68-79.
Eleven previously undescribed compounds, including four benzophenones (garciosones A-D), four xanthones (garciosones E-H) and three biphenyls (garciosines A-C), along with eighteen known compounds were isolated from the stems, leaves and twigs of Garcinia speciosa Wall. (Clusiaceae). Their structures were established by extensive spectroscopic analysis. For garciosines A-C, the structures were confirmed by single crystal X-ray diffraction analysis. Most of the isolated compounds were evaluated for their cytotoxic activity and anti-HIV-1 activity using the syncytium inhibition assay and HIV-1 reverse transcriptase (RT) assay. The known compounds, 4,6,3',4'-tetrahydroxy-2-methoxybenzophenone and Macluraxanthone, displayed significant cytotoxic activity with the ED50 in the range of 1.85-11.76 muM. 1,5-Dihydroxyxanthone exhibited the most potent anti-HIV activity against syncytium formation with EC50 < 17.13 muM (SI > 25.28) and 2-(3,3-dimethylallyl)-1,3,7-trihydroxyxanthone was the most active compound in the HIV-1 reverse transcriptase assay with IC50 value of 58.24 muM. Structure-activity relationship of some isolated compounds were also discussed.
Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia.[Pubmed:26957972]
Afr Health Sci. 2015 Sep;15(3):835-40.
BACKGROUND: Plasmodium falciparum, one of the causative agents of malaria, has high adaptability through mutation and is resistant to many types of anti-malarial drugs. This study presents an in vitro assessment of the antiplasmodial activity of some phenolic compounds isolated from plants of the genus Allanblackia. METHODS: Tests were performed on well plates filled with a fixed parasitized erythrocytes volume. Compounds to be tested were then added in wells. After incubation, tritiated hypoxanthine is added and the plates were returned to the incubator. After thawing, the nucleic acids are collected. Inhibitory Concentration 50 (IC50) was determined by linear interpolation. RESULTS: From Allanblackia floribunda, have been isolated and characterized 1,7-dihydroxyxanthone 1, Macluraxanthone 4, morelloflavone 9, Volkensiflavone 10 and morelloflavone 7-O-glucoside 11; from Allanblackia monticola, alpha-mangosine 2, rubraxanthone 3, allaxanthone C 5, norcowanine 6, tovophiline A 7, allaxanthone B 8 and from Allanblackia gabonensis, 1,7-dihydroxyxanthone 1. Six of them were evaluated for their antimalarial properties. The most active compound, Macluraxanthone, presented a very interesting activity, with an IC50 of 0.36 and 0.27 microg/mL with the F32 and FcM29 strains respectively. CONCLUSION: This work confirms that species of Allanblackia genus are medicinally important plants containing many biologically active compounds that can be used effectively as antiplasmodial.
Antiproliferative xanthone derivatives from Calophyllum inophyllum and Calophyllum soulattri.[Pubmed:25730799]
Pak J Pharm Sci. 2015 Mar;28(2):425-9.
Structure-activity relationships of eleven xanthones were comparatively predicted for four cancer cell lines after the compounds were subjected to antiproliferative assay against B-lymphocyte cells (Raji), colon carcinoma cells (LS174T), human neuroblastoma cells (IMR-32) and skin carcinoma cells (SK-MEL-28). The eleven chemical constituents were obtained naturally from the stem bark of Calophyllum inophyllum and Calophyllum soulattri. Inophinnin (1) and inophinone (2) were isolated from Calophyllum inophyllum while soulattrin (3) and phylattrin (4) were found from Calophyllum soulattri. The other xanthones were from both Calophyllum sp. and they are pyranojacareubin (5), rheediaxanthone A (6), Macluraxanthone (7), 4-hydroxyxanthone (8), caloxanthone C (9), brasixanthone B (10) and trapezifolixanthone (11). Compound 3 was found to be the most cytotoxic towards all the cancer cell lines with an IC50 value of 1.25mug/mL while the simplest xanthone, compound 8 was inactive.
Calophyllum inophyllum and Calophyllum soulattri source of anti-proliferative xanthones and their structure-activity relationships.[Pubmed:25229947]
Nat Prod Res. 2015;29(1):98-101.
Extensive chromatographic isolation and purification of the extracts of the stem bark of Calophyllum inophyllum and Calophyllum soulattri have resulted in 11 xanthones. C. inophyllum gave inophinnin (1), inophinone (2), pyranojacareubin (5), rheediaxanthone A (6), Macluraxanthone (7) and 4-hydroxyxanthone (8), while C. soulattri afforded soulattrin (3), phylattrin (4), caloxanthone C (9), brasixanthone B (10) and trapezifolixanthone (11). The structures of these compounds were determined on the basis of spectroscopic analyses such as 1D and 2D NMR, GC-MS, IR and UV. Cytotoxicity screening (MTT assay) carried out in vitro on all the xanthones using five human cancer cell lines indicated good activities for some of these xanthones. The structure-activity relationship study revealed that the inhibitory activities exhibited by these xanthone derivatives to be closely related to the existence and nature of the pyrano and the prenyl substituent groups on their skeleton.
Cytotoxicity and structure-activity relationships of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora towards nine human cancer cell lines.[Pubmed:23381024]
Molecules. 2013 Feb 4;18(2):1985-94.
The cytotoxic structure-activity relationships among a series of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora were studied. Eleven xanthone derivatives identified as mesuarianone (1), mesuasinone (2), mesuaferrin A (3), mesuaferrin B (4), mesuaferrin C (5), 6-deoxyjacareubin (6), caloxanthone C (7), Macluraxanthone (8), 1,5-dihydroxyxanthone (9), tovopyrifolin C (10) and alpha-mangostin (11) were isolated from the three Mesua species. The human cancer cell lines tested were Raji, SNU-1, K562, LS-174T, SK-MEL-28, IMR-32, HeLa, Hep G2 and NCI-H23. Mesuaferrin A (3), Macluraxanthone (8) and alpha-mangostin (11) showed strong cytotoxicities as they possess significant inhibitory effects against all the cell lines. The structure-activity relationship (SAR) study revealed that the diprenyl, dipyrano and prenylated pyrano substituent groups of the xanthone derivatives contributed towards the cytotoxicities.
Anti-metastatic effects on B16F10 melanoma cells of extracts and two prenylated xanthones isolated from Maclura amboinensis Bl roots.[Pubmed:22994788]
Asian Pac J Cancer Prev. 2012;13(7):3519-28.
Inhibitory effects of Maclura amboinenesis Bl, one plant used traditionally for the treatment of cancers, on metastatic potential of highly metastatic B16F10 melanoma cells were investigated in vitro. Cell proliferation was assessed using the MTT colorimetric assay. Details of metastatic capabilities including invasion, migration and adhesion of B16F10 melanoma cells were examined by Boyden Chamber invasion and migration, scratch motility and cell attachment assays, respectively. The results demonstrated that n-hexane and chloroform extracts exhibited potent anti-proliferative effects (p<0.01), whereas the methanol and aqueous extracts had less pronounced effects after 24 h exposure. Bioactivity-guided chromatographic fractionation of both active n-hexane and chloroform extracts led to the isolation of two main prenylated xanthones and characterization as Macluraxanthone and gerontoxanthone-I, respectively, their structures being identified by comparison with the spectral data. Interestingly, both exhibited potent effective effects. At non-toxic effective doses, n-hexane and chloroform extracts (10 and 30 mug/ml) as well as Macluraxanthone and gerontoxanthone-I (3 and 10 muM) significantly inhibited B16F10 cell invasion, to a greater extent than 10 muM doxorubicin, while reducing migration of cancer cells without cellular cytotoxicity. Moreover, exposure of B16F10 melanoma cells to high concentrations of chloroform (30 mug/ml) and geratoxanthone-I (20 muM) for 24 h resulted in delayed adhesion and retarded colonization. As insights into mechanisms of action, typical morphological changes of apoptotic cells e.g. membrane blebbing, chromatin condensation, nuclear fragmentation, apoptotic bodies and loss of adhesion as well as cell cycle arrest in the G1 phase with increase of sub-G1 cell proportions, detected by Hoechst 33342 staining and flow cytometry were observed, suggesting DNA damage and subsequent apoptotic cell death. Taken together, our findings indicate for the first time that active n-hexane and chloroform extracts as well as Macluraxanthone and gerontoxanthone-I isolated from Maclura amboinensis Bl. roots affect multistep of cancer metastasis processes including proliferation, adhesion, invasion and migration, possibly through induction of apoptosis of highly metastatic B16F10 melanoma cells. Based on these data, M. amboinensis Bl. represents a potential candidate novel chemopreventive and/or chemotherapeutic agent. Additionally, they also support its ethno-medicinal usage for cancer prevention and/or chemotherapy.
Phylattrin, a new cytotoxic xanthone from Calophyllum soulattri.[Pubmed:22781442]
Molecules. 2012 Jul 10;17(7):8303-11.
Our continuing studies on secondary metabolites from the stem bark of Calophyllum soulattri has led to the isolation of another new diprenylated xanthone, phylattrin (1), in addition to five other xanthones and two common sterols. The xanthones are soulattrin (2), caloxanthone C (3), Macluraxanthone (4), brasixanthone B (5) and trapezifolixanthone (6) while the sterols are stigmasterol (7) and beta-sitosterol (8). The structures of these compounds were determined on the basis of spectroscopic analyses such as 1D and 2D-NMR, HRESIMS, IR and UV. Compounds 1-7 exhibited moderate cytotoxic activities against SNU-1, HeLa, Hep G2, NCI-H23, K562, Raji, LS174T, IMR-32 and SK-MEL-28 cells.
Soulamarin, a new coumarin from stem bark of Calophyllum soulattri.[Pubmed:22113580]
Molecules. 2011 Nov 23;16(11):9721-7.
The extracts of the stem bark of Calophyllum soulattri gave a new pyranocoumarin, soulamarin (1), together with five other xanthones caloxanthone B (2), caloxanthone C (3), Macluraxanthone (4), trapezifolixanthone (5) and brasixanthone B (6) one common triterpene, friedelin (7), and the steroidal triterpene stigmasterol (8). The structures of these compounds were established based on spectral evidence (1D and 2D NMR).
A new furanoxanthone from the stem bark of Calophyllum inophyllum.[Pubmed:21972812]
J Asian Nat Prod Res. 2011 Oct;13(10):956-60.
The stem bark extracts of Calophyllum inophyllum furnished one new furanoxanthone, inophinnin (1), in addition to inophyllin A (2), Macluraxanthone (3), pyranojacareubin (4), 4-hydroxyxanthone, friedelin, stigmasterol, and betulinic acid. The structures of these compounds were determined by spectroscopic analysis of 1D and 2D NMR spectral data ((1)H, (13)C, DEPT, COSY, HMQC, and HMBC) while EI-MS gave the molecular mass. The new xanthone, inophinnin (1), exhibited some anti-inflammatory activity in nitric oxide assay.
Antioxidant and antimutagenic polyisoprenylated benzophenones and xanthones from Rheedia acuminata.[Pubmed:21941896]
Nat Prod Commun. 2011 Sep;6(9):1269-74.
Dichloromethane extract of the stem bark of Rheedia acuminata yielded three benzophenones with antioxidant activity, the new one named acuminophenone A (1), guttiferone K (2) and isoxanthochymol (3), along with the known xanthones formoxanthone C (4) and Macluraxanthone (5). The structures were established through interpretation of their spectroscopic data, the stereochemistry of compounds (1) and (2) were resolved by experimental and computational experiments and their antioxidant activities were measured using the DPPH, ABTS and TEAC assays. The antioxidant results showed that metabolites 1, 4 and 5 had a better antioxidant activity than the reference compound quercetin. In addition, we evaluate the mutagenicity and antimutagenicity of the CH2Cl2 extract as well as of the free radical scavenger compounds 1, 4 and 5 by the AMES Salmonella/microsomal test. No mutagenicity was found in the CH2Cl2 extract using Salmonella typhimurium strains TA98, TA100, TA102, TA1537 and TA1538, with or without S9 metabolic activation. The pure compounds neither showed mutagenicity in TA 102 strain and the most important result was the strong reduction of mutagenic effect induced by hydrogen peroxide in S. typhimurium TA102, with or without S9, showed by the compounds 1 (more than 93%) and 4 (more than 88%) at 0.02 microg/plate.
Therapeutic activity of two xanthones in a xenograft murine model of human chronic lymphocytic leukemia.[Pubmed:21138552]
J Hematol Oncol. 2010 Dec 7;3:49.
BACKGROUND: We previously reported that allanxanthone C and Macluraxanthone, two xanthones purified from Guttiferae trees, display in vitro antiproliferative and proapoptotic activities in leukemic cells from chronic lymphocytic leukemia (CLL) and leukemia B cell lines. RESULTS: Here, we investigated the in vivo therapeutic effects of the two xanthones in a xenograft murine model of human CLL, developed by engrafting CD5-transfected chronic leukemia B cells into SCID mice. Treatment of the animals with five daily injections of either allanxanthone C or Macluraxanthone resulted in a significant prolongation of their survival as compared to control animals injected with the solvent alone (p = 0.0006 and p = 0.0141, respectively). The same treatment of mice which were not xenografted induced no mortality. CONCLUSION: These data show for the first time the in vivo antileukemic activities of two plant-derived xanthones, and confirm their potential interest for CLL therapy.
Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies.[Pubmed:19596285]
Chem Biol Interact. 2009 Oct 30;181(3):383-9.
Flavonoids are one of the largest classes of plant secondary metabolites and are known to possess a number of significant biological activities for human health. In this study, we examined in vitro acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities of four flavonoid derivatives--quercetin, rutin, kaempferol 3-O-beta-D-galactoside and Macluraxanthone. The in vitro results showed that quercetin and Macluraxanthone displayed a concentration-dependant inhibition of AChE and BChE. Macluraxanthone showed to be the most potent and specific inhibitor of both the enzymes having the IC(50) values of 8.47 and 29.8 microM, respectively. The enzyme kinetic studies revealed that quercetin inhibited both the enzymes in competitive manner, whereas the mode of inhibition of Macluraxanthone was non-competitive against AChE and competitive against BChE. The inhibitory profiles of the compounds have been compared with standard AChE inhibitor galanthamine. To get insight of the intermolecular interactions, the molecular docking studies of these two compounds were performed at the active site 3D space of both the enzymes, using ICM-Dock module. Docking studies exhibited that Macluraxanthone binds much more tightly with both the enzymes than quercetin. The calculated docking and binding energies also supported the in vitro inhibitory profiles (IC(50) values). Both the compounds showed several strong hydrogen bonds to several important amino acid residues of both the enzymes. A number of hydrophobic interactions could also explain the potency of the compounds to inhibit AChE and BChE.
Constituents of the Cuban endemic species Calophyllum pinetorum.[Pubmed:18553925]
J Nat Prod. 2008 Jul;71(7):1283-6.
A new prenylated xanthone, pinetoxanthone (1), and two new pyranochromanones, pinetoric acid I (2) and pinetoric acid II (3), together with 10 known compounds, namely, the triterpenes friedelin and canophyllol, the xanthone Macluraxanthone, the pyranochromanone derivatives calophyllic acid, isocalophyllic acid, calolongic acid, apetalic acid, and isoapetalic acid, and the flavonoids amentoflavone and apigenin, were isolated from the stem bark and leaves of Calophyllum pinetorum, an endemic species in Cuba. The structures of 1-3 were elucidated by spectroscopic methods including 1D and 2D NMR experiments as well as HRESIMS analysis.


