MaackiainCAS# 19908-48-6 |
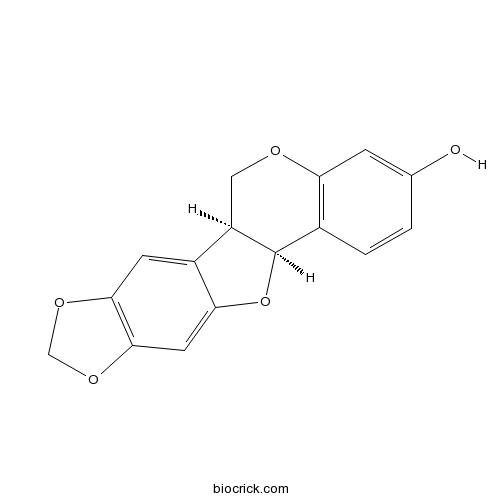
- (-)-Maackiain
Catalog No.:BCN4892
CAS No.:2035-15-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19908-48-6 | SDF | Download SDF |
| PubChem ID | 161298 | Appearance | Powder |
| Formula | C16H12O5 | M.Wt | 284.27 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | C1C2C(C3=C(O1)C=C(C=C3)O)OC4=CC5=C(C=C24)OCO5 | ||
| Standard InChIKey | HUKSJTUUSUGIDC-BDJLRTHQSA-N | ||
| Standard InChI | InChI=1S/C16H12O5/c17-8-1-2-9-12(3-8)18-6-11-10-4-14-15(20-7-19-14)5-13(10)21-16(9)11/h1-5,11,16-17H,6-7H2/t11-,16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Maackiain is an antimicrobial compound (phytoalexins), it has anticancer effects, it induces apoptosis and growth suppression. Maackiain also shows strong larvicidal activity (LC50 = 21.95 ± 1.34 ug/mL). |
| Targets | Antifection |
| In vitro | Extract of Bowdichia virgilioides and maackiain as larvicidal agent against Aedes aegypti mosquito.[Pubmed: 25819294]Exp Parasitol. 2015 Jun;153:160-4.
Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells.[Pubmed: 15547735]Oncol Rep. 2004 Dec;12(6):1183-8.
|
| Kinase Assay | Three Genes for Metabolism of the Phytoalexin Maackiain in the Plant Pathogen Nectria haematococca: Meiotic Instability and Relationship to a New Gene for Pisatin Demethylase.[Pubmed: 16348671 ]Appl Environ Microbiol. 1992 Mar;58(3):801-8.Some isolates of the plant-pathogenic fungus Nectria haematococca mating population (MP) VI metabolize Maackiain and medicarpin, two antimicrobial compounds (phytoalexins) synthesized by chickpea (Cicer arietinum L.). The enzymatic modifications by the fungus convert the phytoalexins to less toxic derivatives, and this detoxification has been proposed to be important for pathogenesis on chickpea. |

Maackiain Dilution Calculator

Maackiain Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5178 mL | 17.5889 mL | 35.1778 mL | 70.3556 mL | 87.9446 mL |
| 5 mM | 0.7036 mL | 3.5178 mL | 7.0356 mL | 14.0711 mL | 17.5889 mL |
| 10 mM | 0.3518 mL | 1.7589 mL | 3.5178 mL | 7.0356 mL | 8.7945 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7036 mL | 1.4071 mL | 1.7589 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7036 mL | 0.8794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bakkenolide A
Catalog No.:BCN5402
CAS No.:19906-72-0
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Humulene epoxide II
Catalog No.:BCN4873
CAS No.:19888-34-7
- Alisol A
Catalog No.:BCN3455
CAS No.:19885-10-0
- H-Phe(2-F)-OH
Catalog No.:BCC3222
CAS No.:19883-78-4
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- T 98475
Catalog No.:BCC7395
CAS No.:199119-18-1
- Furanodiene
Catalog No.:BCN6454
CAS No.:19912-61-9
- Chamigrenal
Catalog No.:BCN7847
CAS No.:19912-84-6
- Futoenone
Catalog No.:BCN6408
CAS No.:19913-01-0
- Enniatin B1
Catalog No.:BCN4853
CAS No.:19914-20-6
- Boc-D-N-Me-Ala-OH
Catalog No.:BCC3211
CAS No.:19914-38-6
- O6-Benzylguanine
Catalog No.:BCC6485
CAS No.:19916-73-5
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
A gene for maackiain detoxification from a dispensable chromosome of Nectria haematococca.[Pubmed:8709942]
Mol Gen Genet. 1996 Jun 24;251(4):397-406.
In Nectria haematococca the MAK1 gene product converts a chick-pea (Cicer arietinum) phytoalexin, Maackiain, into a less toxic compound. The presence of MAK1 in this fungal pathogen is also correlated with high virulence on chick-pea. Previous genetic analysis suggested that MAK1 is located on a meiotically unstable, dispensable chromosome. The unstable nature of this chromosome facilitated MAK1 cloning by allowing us to identify a subset of genomic cosmid clones likely to contain MAK1. Truncated forms of the chromosome, generated during meiosis, were isolated from strains either able (Mak+) or unable (Mak-) to metabolize Maackiain and used to probe a chromosome-specific cosmid library. Only clones that hybridized exclusively to the chromosome from the Mak+ strain were then screened for their ability to transform a Mak- isolate to the Mak+ phenotype. A 2.7 kb HindIII-PstI fragment was subcloned from a cosmid conferring MAK1 activity, and its nucleotide sequence determined. Because MAK1 transcription is not induced strongly by Maackiain, a reverse transcriptase-polymerase chain reaction was required to detect MAK1 transcription in a Mak+ strain, and to isolate MAK1 cDNA fragments. Comparison of the genomic and cDNA sequences of MAK1 revealed the presence of three introns and an open reading frame encoding a protein 460 amino acids in length. Two diagnostic domains in its deduced amino acid sequence suggest MAK1 encodes a flavin-containing mono-oxygenase. MAK1 is the first gene encoding Maackiain detoxification to be cloned, and is the second functional gene cloned from this dispensable chromosome. Southern analysis of genomic DNA from ascospore isolates containing MAK2, MAK3, and MAK4 indicated that MAK1 is not homologous to other known maackianin-detoxifying genes.
Three Genes for Metabolism of the Phytoalexin Maackiain in the Plant Pathogen Nectria haematococca: Meiotic Instability and Relationship to a New Gene for Pisatin Demethylase.[Pubmed:16348671]
Appl Environ Microbiol. 1992 Mar;58(3):801-8.
Some isolates of the plant-pathogenic fungus Nectria haematococca mating population (MP) VI metabolize Maackiain and medicarpin, two antimicrobial compounds (phytoalexins) synthesized by chickpea (Cicer arietinum L.). The enzymatic modifications by the fungus convert the phytoalexins to less toxic derivatives, and this detoxification has been proposed to be important for pathogenesis on chickpea. In the present study, loci controlling Maackiain metabolism (Mak genes) were identified by crosses among isolates of N. haematococca MP VI that differed in their ability to metabolize the phytoalexin. Strains carrying Mak1 or Mak2 converted Maackiain to 1a-hydroxyMaackiain, while those with Mak3 converted it to 6a-hydroxyMaackiain. Mak1 and Mak2 were unusual in that they often failed to be inherited by progeny. Mak1 was closely linked to Pda6, a new member in a family of genes in N. haematococca MP VI that encode enzymes for detoxification of pisatin, the phytoalexin synthesized by garden pea. Like Mak1, Pda6 was also transmitted irregularly to progeny. Although the unusual meiotic behaviors of some Mak genes complicate genetic analysis, identification of these genes should afford a more through evaluation of the role of phytoalexin detoxification in the pathogenesis of N. haematococca MP VI on chickpea.
Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells.[Pubmed:15547735]
Oncol Rep. 2004 Dec;12(6):1183-8.
We have investigated the effects of Maackiain and trifolirhizin (Maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) on DNA of human promyelotic HL-60 leukemia cells. It was found that extent of induction of apoptosis by Maackiain was larger than that by trifolirhizin in human leukemia HL-60 cells. Morphological changes showing apoptotic bodies were observed in the HL-60 cells treated with Maackiain and trifolirhizin. The fragmentations of DNA by Maackiain and trifolirhizin to oligonucleosomal-sized fragments that is a characteristic of apoptosis was observed to be concentration- and time-dependent in the HL-60 cells. The data of the present study show that the suppressions by Maackiain and trifolrhizin of growth of the HL-60 cells result from the induction of apoptosis by these compounds, and that the extent of growth suppression and induction of apoptosis by Maackiain was greater than that by the glycoside (trifolirhizin).
Extract of Bowdichia virgilioides and maackiain as larvicidal agent against Aedes aegypti mosquito.[Pubmed:25819294]
Exp Parasitol. 2015 Jun;153:160-4.
The larvicidal activities of extracts of three hardwood species (Hymenaea stigonorcapa, Anadenanthera colubrina and Bowdichia virgilioides) against 4th instar larvae of Aedes aegypti were evaluated using WHO guidelines. Extracts of H. stignocarpa and A. colubrina showed weak activity. The highest larvicidal effect was obtained with the cyclohexane extract of the heartwood of B. virgilioides, which caused 100% mortality at concentrations at 50 and 100 microg/mL. Fraction toluene/EtOAc (8:2) from this extract showed larvicidal activity (LC(5)(0) = 34.90 +/- 1.27 microg/mL). A mixture of two compounds identified as medicarpin and Maackiain exhibited a very good larvicidal activity (sub-fraction 2, LC(5)(0) = 17.5 +/- 1.87 microg/mL) and Maackiain showed to be a strong larvicidal compound (LC(5)(0) = 21.95 +/- 1.34 microg/mL). This result can be of value in the search for new natural larvicidal compounds from other hardwood plant extracts and presents the first report of B. virgilioides being used to control a mosquito vector.


