Lycopsamine N-oxideCAS# 95462-15-0 |
- Indicine N-oxide
Catalog No.:BCN1996
CAS No.:41708-76-3
- Intermedine N-oxide
Catalog No.:BCN8931
CAS No.:95462-14-9
- Echinatine N-oxide
Catalog No.:BCN0318
CAS No.:20267-93-0
- Rinderine N-oxide
Catalog No.:BCN0364
CAS No.:137821-16-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 95462-15-0 | SDF | Download SDF |
| PubChem ID | 340067 | Appearance | White powder |
| Formula | C15H25NO6 | M.Wt | 315.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
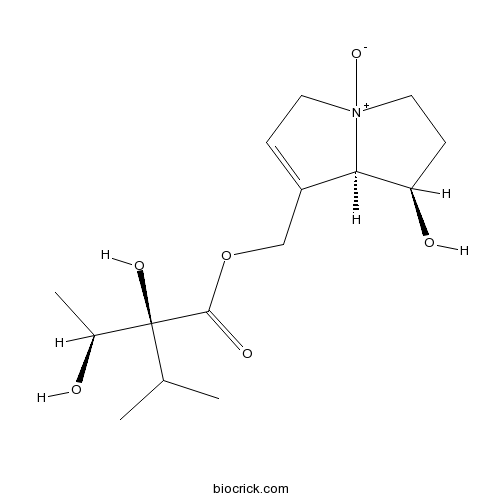
| Chemical Name | [(7R,8R)-7-hydroxy-4-oxido-5,6,7,8-tetrahydro-3H-pyrrolizin-4-ium-1-yl]methyl (2S)-2-hydroxy-2-[(1S)-1-hydroxyethyl]-3-methylbutanoate | ||
| SMILES | CC(C)C(C(C)O)(C(=O)OCC1=CC[N+]2(C1C(CC2)O)[O-])O | ||
| Standard InChIKey | DNAWGBOKUFFVMB-FVZLBROTSA-N | ||
| Standard InChI | InChI=1S/C15H25NO6/c1-9(2)15(20,10(3)17)14(19)22-8-11-4-6-16(21)7-5-12(18)13(11)16/h4,9-10,12-13,17-18,20H,5-8H2,1-3H3/t10-,12+,13+,15-,16?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lycopsamine N-oxide Dilution Calculator

Lycopsamine N-oxide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1706 mL | 15.8529 mL | 31.7058 mL | 63.4115 mL | 79.2644 mL |
| 5 mM | 0.6341 mL | 3.1706 mL | 6.3412 mL | 12.6823 mL | 15.8529 mL |
| 10 mM | 0.3171 mL | 1.5853 mL | 3.1706 mL | 6.3412 mL | 7.9264 mL |
| 50 mM | 0.0634 mL | 0.3171 mL | 0.6341 mL | 1.2682 mL | 1.5853 mL |
| 100 mM | 0.0317 mL | 0.1585 mL | 0.3171 mL | 0.6341 mL | 0.7926 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Echimidine N-oxide
Catalog No.:BCN8950
CAS No.:41093-89-4
- Homatropine
Catalog No.:BCN8948
CAS No.:87-00-3
- Merepoxine
Catalog No.:BCN8946
CAS No.:115777-94-1
- Indicine hydrochloride
Catalog No.:BCN8945
CAS No.:1195140-94-3
- Heliosupine N-oxide
Catalog No.:BCN8943
CAS No.:31701-88-9
- Glucoraphenin
Catalog No.:BCN8942
CAS No.:108844-81-1
- 6-Hydroxytropinone
Catalog No.:BCN8940
CAS No.:5932-53-6
- Glucohesperin
Catalog No.:BCN8939
CAS No.:33049-17-1
- Sceleratine N-oxide
Catalog No.:BCN8938
CAS No.:103184-92-5
- Senecivernine N-oxide
Catalog No.:BCN8937
CAS No.:101687-28-9
- Riddelline N-oxide
Catalog No.:BCN8936
CAS No.:75056-11-0
- 7-O-Acetyllycopsamine N-oxide
Catalog No.:BCN8935
CAS No.:685132-58-5
- Usaramine N-oxide
Catalog No.:BCN8952
CAS No.:117020-54-9
- Scopolamine N-oxide hydrobromide
Catalog No.:BCN8953
CAS No.:6106-81-6
- Noratropine
Catalog No.:BCN8955
CAS No.:16839-98-8
- Glucoraphasatin
Catalog No.:BCN8957
CAS No.:28463-23-2
- Glucobrassicin
Catalog No.:BCN8958
CAS No.:143231-38-3
- Glucocheirolin
Catalog No.:BCN8959
CAS No.:15592-36-6
- Sinalbin potassium salt
Catalog No.:BCN8960
CAS No.:16411-05-5
- Gluconasturtiin
Catalog No.:BCN8961
CAS No.:18425-76-8
- Glucohirsutin
Catalog No.:BCN8962
CAS No.:21973-60-4
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
Absorption difference between hepatotoxic pyrrolizidine alkaloids and their N-oxides - Mechanism and its potential toxic impact.[Pubmed:31759111]
J Ethnopharmacol. 2020 Mar 1;249:112421.
ETHNOPHARMACOLOGICAL RELEVANCE: Pyrrolizidine alkaloids (PAs) are a group of phytotoxins widely present in about 3% of flowering plants. Many PA-containing herbal plants can cause liver injury. Our previous studies demonstrated that PA N-oxides are also hepatotoxic, with toxic potency much lower than the corresponding PAs, due to significant differences in their toxicokinetic fates. AIM OF STUDY: This study aimed to investigate the oral absorption of PAs and PA N-oxides for better understanding of their significant differences in toxicokinetics and toxic potency. MATERIALS AND METHODS: The oral absorption of PAs and PA N-oxides in rats and in rat in situ single pass intestine perfusion model was investigated. The intestinal permeability and absorption mechanisms of five pairs of PAs and PA N-oxides were evaluated by using Caco-2 monolayer model. RESULTS: The plasma concentrations of total PAs and PA N-oxides within 0-60 min were significantly lower in rats orally treated with a PA N-oxide-containing herbal alkaloid extract than with a PA-containing herbal alkaloid extract at the same dose, indicating that the absorption of PA N-oxides was lower than that of PAs. Using the rat in situ single pass intestine perfusion model, less cumulative amounts of retrorsine N-oxide in mesenteric blood were observed compared to that of retrorsine. In Caco-2 monolayer model, all five PAs showed absorption with Papp AtoB values [(1.43-16.26) x 10(-6) cm/s] higher than those of corresponding N-oxides with Papp AtoB values lower than 1.35 x 10(-6) cm/s. A further mechanistic study demonstrated that except for senecionine N-oxide, retrorsine N-oxide, and Lycopsamine N-oxide, all PAs and PA N-oxides investigated were absorbed via passive diffusion. While, for these 3 PA N-oxides, in addition to passive diffusion as their primary transportation, efflux transporter-mediated active transportation was also involved but to a less extent with the efflux ratio of 2.31-3.41. Furthermore, a good correlation between lipophilicity and permeability of retronecine-type PAs and their N-oxides with absorption via passive diffusion was observed, demonstrating that PAs have a better oral absorbability than that of the corresponding PA N-oxides. CONCLUSION: We discovered that among many contributors, the lower intestinal absorption of PA N-oxides was the initiating contributor that caused differences in toxicokinetics and toxic potency between PAs and PA N-oxides.


