LicoflavonolCAS# 60197-60-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60197-60-6 | SDF | Download SDF |
| PubChem ID | 5481964 | Appearance | Powder |
| Formula | C20H18O6 | M.Wt | 354.35 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
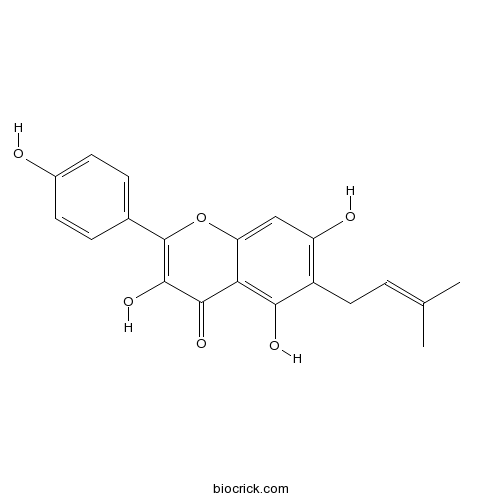
| Chemical Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-6-(3-methylbut-2-enyl)chromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1O)C(=O)C(=C(O2)C3=CC=C(C=C3)O)O)O)C | ||
| Standard InChIKey | TVMHBSODLWMMMV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H18O6/c1-10(2)3-8-13-14(22)9-15-16(17(13)23)18(24)19(25)20(26-15)11-4-6-12(21)7-5-11/h3-7,9,21-23,25H,8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Licoflavonol exhibits a strong inhibitory effect on the secretion of the SPI-1 effector proteins via regulating the transcription of the SicA/InvF genes, and the transportation of the effector protein SipC, thus, it is a novel natural inhibitor of Salmonella T3SS, could be a promising candidate for novel type of anti-virulence drugs. |
| Targets | Antifection |

Licoflavonol Dilution Calculator

Licoflavonol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8221 mL | 14.1103 mL | 28.2207 mL | 56.4414 mL | 70.5517 mL |
| 5 mM | 0.5644 mL | 2.8221 mL | 5.6441 mL | 11.2883 mL | 14.1103 mL |
| 10 mM | 0.2822 mL | 1.411 mL | 2.8221 mL | 5.6441 mL | 7.0552 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5644 mL | 1.1288 mL | 1.411 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5644 mL | 0.7055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl 15-hydroxy-7-oxodehydroabietate
Catalog No.:BCN7674
CAS No.:60188-95-6
- Corypalmine
Catalog No.:BCN4111
CAS No.:6018-40-2
- Arecaidine hydrochloride
Catalog No.:BCN8530
CAS No.:6018-28-6
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- TWS119
Catalog No.:BCC4512
CAS No.:601514-19-6
- 11,12-Dihydro-7-hydroxyhedychenone
Catalog No.:BCN7382
CAS No.:60149-07-7
- Gabapentin
Catalog No.:BCC3783
CAS No.:60142-96-3
- Gabapentin HCl
Catalog No.:BCC4502
CAS No.:60142-95-2
- Cephaelin dihydrobromide
Catalog No.:BCC8142
CAS No.:6014-81-9
- Coulteropine
Catalog No.:BCN7420
CAS No.:6014-62-6
- Cucurbitacin S
Catalog No.:BCN2676
CAS No.:60137-06-6
- Ercalcitriol
Catalog No.:BCC1556
CAS No.:60133-18-8
- Taspine
Catalog No.:BCN6956
CAS No.:602-07-3
- Thiocolchicoside
Catalog No.:BCN8442
CAS No.:602-41-5
- 1,2-dihydroxy-3-methyl-anthracene-9,10-dione
Catalog No.:BCN1404
CAS No.:602-63-1
- Coptisine chloride
Catalog No.:BCN6321
CAS No.:6020-18-4
- Altamycin A
Catalog No.:BCN1823
CAS No.:60202-22-4
- Oleanolic acid-3-O-beta-D-glucopyranosyl (1→2)-alpha-L-arabinopyranoside
Catalog No.:BCN1406
CAS No.:60213-69-6
- AZD-5438
Catalog No.:BCC3689
CAS No.:602306-29-6
- Tetrahydropalmatine Hydrochloride
Catalog No.:BCN8335
CAS No.:6024-85-7
- (±)-Nipecotic acid
Catalog No.:BCC6576
CAS No.:60252-41-7
- Ethyl(1-hydroxy-4-oxocyclohexa-2,5-dien-1-yl)acetate
Catalog No.:BCN1405
CAS No.:60263-06-1
- H-D-HoSer-OH
Catalog No.:BCC3243
CAS No.:6027-21-0
- Aspalathin
Catalog No.:BCC8122
CAS No.:6027-43-6
Metabolites identification of bioactive licorice compounds in rats.[Pubmed:26311472]
J Pharm Biomed Anal. 2015 Nov 10;115:515-22.
Licorice (Glycyrrhiza uralensis Fisch.) is one of the most popular herbal medicines worldwide. This study aims to identify the metabolites of seven representative bioactive licorice compounds in rats. These compounds include 22beta-acetoxyl glycyrrhizin (1), Licoflavonol (2), licoricidin (3), licoisoflavanone (4), isoglycycoumarin (5), semilicoisoflavone B (6), and 3-methoxy-9-hydroxy-pterocarpan (7). After oral administration of 250mg/kg of 1 or 40mg/kg of 2-7 to rats, a total of 16, 43 and 31 metabolites were detected in the plasma, urine and fecal samples, respectively. The metabolites were characterized by HPLC/DAD/ESI-MS(n) and LC/IT-TOF-MS analyses. Particularly, two metabolites of 1 were unambiguously identified by comparing with reference standards, and 22beta-acetoxyl glycyrrhizin-6''-methyl ester (1-M2) is a new compound. Compound 1 could be readily hydrolyzed to eliminate the glucuronic acid residue. The phenolic compounds (4-7) mainly undertook phase II metabolism (glucuronidation or sulfation). Most phenolic compounds with an isoprenyl group (chain or cyclized, 2-5) could also undertake hydroxylation reaction. This is the first study on in vivo metabolism of these licorice compounds.
Simultaneous determination of five minor coumarins and flavonoids in Glycyrrhiza uralensis by solid-phase extraction and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry.[Pubmed:24496986]
Planta Med. 2014 Feb;80(2-3):237-42.
Minor phenolic compounds in licorice (Glycyrrhiza uralensis) have recently been proved for diverse bioactivities and favorable bioavailability, indicating that they may play an important role in the therapeutic effects or herb-drug interactions of licorice. However, so far, their abundance in licorice remains unknown. In this study, a reliable solid-phase extraction coupled with a high-performance liquid chromatography and diode array detection method was established to determine the minor phenolic compounds in licorice. The analytes were enriched by a three-step solid-phase extraction method, and then separated on a YMC ODS-A column by gradient elution. Five coumarins and flavonoids were identified by electrospray ionization tandem mass spectrometry, and then quantified using high-performance liquid chromatography and diode array detection. The amounts of glycycoumarin, dehydroglyasperin C, glycyrol, Licoflavonol, and glycyrin in G. uralensis were 0.81 +/- 0.28, 1.25 +/- 0.59, 0.20 +/- 0.08, 0.12 +/- 0.04, and 0.17 +/- 0.08 mg/g, respectively. Abundances of these compounds in other Glycyrrhiza species (G. glabra, G. inflata, and G. yunnanesis) were remarkably lower than G. uralensis.
Licoflavonol is an inhibitor of the type three secretion system of Salmonella enterica serovar Typhimurium.[Pubmed:27387231]
Biochem Biophys Res Commun. 2016 Sep 2;477(4):998-1004.
As an important food-borne human pathogen, Salmonella enterica serovar Typhimurium depends on its type III secretion system (T3SS) as a major virulence factor to cause disease all over the world. The T3SS secretes effector proteins to facilitate invasion into host cells. In this study, twenty prenylated flavonoids (1-20) were screened for their anti-T3SS activity, revealing that several analogs exhibited strong inhibitory effects on the secretion of Salmonella pathogenicity island 1 (SPI-1)-associated effector proteins without affecting the growth of bacteria and the secretion of the flagellar protein FliC. Among the flavonoids 1-20, Licoflavonol (20) exhibited a strong inhibitory effect on the secretion of the SPI-1 effector proteins via regulating the transcription of the SicA/InvF genes, and the transportation of the effector protein SipC. In summary, Licoflavonol, a novel natural inhibitor of Salmonella T3SS, could be a promising candidate for novel type of anti-virulence drugs.


