LevetiracetamAntiepileptic drug CAS# 102767-28-2 |
- Alarelin Acetate
Catalog No.:BCC1336
CAS No.:79561-22-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102767-28-2 | SDF | Download SDF |
| PubChem ID | 441341 | Appearance | Powder |
| Formula | C8H14N2O2 | M.Wt | 170.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Keppra, UCB L059 | ||
| Solubility | H2O : ≥ 85 mg/mL (499.38 mM) DMSO : ≥ 85 mg/mL (499.38 mM) *"≥" means soluble, but saturation unknown. | ||
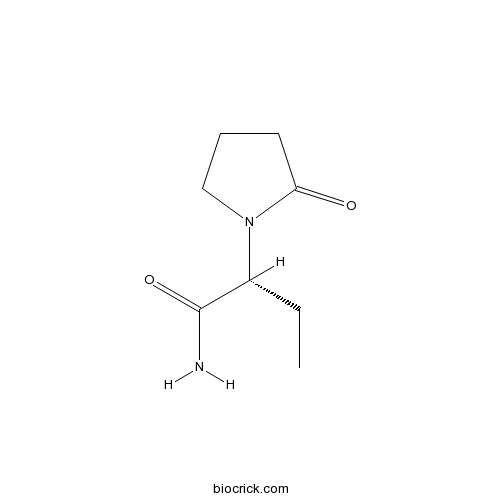
| Chemical Name | (2R)-2-(2-oxopyrrolidin-1-yl)butanamide | ||
| SMILES | CCC(C(=O)N)N1CCCC1=O | ||
| Standard InChIKey | HPHUVLMMVZITSG-ZCFIWIBFSA-N | ||
| Standard InChI | InChI=1S/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)/t6-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Levetiracetam(UCB L059) is a novel anticonvulsant with antihyperalgesic efficacy in inflammatory pain. Target: Calcium Channel Levetiracetam is used to control some types of seizures in patients with epilepsy. This medicine cannot cure epilepsy and will only work to control seizures for as long as you continue to use it. The exact mechanism for levetiracetam is unknown. However, the drug binds to a synaptic vesicle protein, SV2A, which is believed to impede nerve conduction across synapses [1]. Levetiracetam (10-200 mg/kg), ibuprofen (12.5-100 mg/kg), celecoxib (3.75-30 mg/kg), paracetamol (50-200 mg/kg), caffeine (15-100 mg/kg), and 2-drug combinations of levetiracetam with analgesics/caffeine produced a significant, dose-dependent reduction of inflammatory hyperalgesia. Isobolographic analysis revealed that levetiracetam exerts a synergistic interaction with analgesics, with approximately 7-, 9-, and 11-fold reduction of doses of both drugs in combination of levetiracetam with paracetamol, celecoxib, and ibuprofen, respectively. Analysis of the log dose-response curves for levetiracetam (1-50 mg/kg) in the presence of caffeine (10 mg/kg) and levetiracetam applied alone also revealed a synergistic interaction. Levetiracetam's ED50 in the presence of caffeine was reduced approximately 11-fold [2]. Clinical indications: Epilepsy; Social phobia FDA Approved Date: November 2008 Toxicity: depression; hallucinations; suicidal thoughts | |||||

Levetiracetam Dilution Calculator

Levetiracetam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8751 mL | 29.3755 mL | 58.751 mL | 117.5019 mL | 146.8774 mL |
| 5 mM | 1.175 mL | 5.8751 mL | 11.7502 mL | 23.5004 mL | 29.3755 mL |
| 10 mM | 0.5875 mL | 2.9375 mL | 5.8751 mL | 11.7502 mL | 14.6877 mL |
| 50 mM | 0.1175 mL | 0.5875 mL | 1.175 mL | 2.35 mL | 2.9375 mL |
| 100 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.175 mL | 1.4688 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Levetiracetam (LEV) is a newly developed antiepileptic drug (AED) with a broad spectrum of efficacy against partial and generalized seizures. [1]
The mode of action of LEV seems to be different from other AED. Synaptic vesicle protein 2A which can block calcium release from intraneuronal stores is shown to be the binding site of levetiracetam. Thus, it works against the negative modulators of gamma-aminobutyric acid and glycin-gated currents. Moreover, LEV does not have the ablility to induce cytochrome P450 isoenzyme.[2]
A study has shown that an oral dose of LEV can’t bind to plasma proteins, while no hepatic metabolism was observed. One-third of the metabolized drug was found to be hydrolyzed and the others were excreted unchanged in the urine.[3]
References:
[1] Nabil J. Azar, Patsy Aune. Acute pancreatitis and elevated liver transaminases after rapid titration of oral levetiracetam. Journal of Clinical Neuroscience. June 2014. 21(6): 1053-1054.
[2] Xiao-Qiao Chen, Wei-Na Zhang, Zhi-Xian Yang, Meng Zhao, Fang-Cheng Cai, Shao-Ping Huang, Li Gao, Bao-Dong Pang, Xi Chen, Li-Ping Zou. Efficacy of Levetiracetam in Electrical Status Epilepticus During Sleep of Children: A Multicenter Experience. Pediatric Neurology. March 2014. 50(3): 243-249.
[3] Shahnaz Akhtar Chaudhry, Geert’t Jong, Gideon Koren. The fetal safety of Levetiracetam: A systematic review. Reproductive Toxicology. July 2014. 46: 40-45.
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- RO-3
Catalog No.:BCC7548
CAS No.:1026582-88-6
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- Pantoprazole
Catalog No.:BCC5432
CAS No.:102625-70-7
- Saprorthoquinone
Catalog No.:BCN3147
CAS No.:102607-41-0
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
- Methyl lucidente G
Catalog No.:BCN8269
CAS No.:102607-20-5
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- VUF 10460
Catalog No.:BCC6285
CAS No.:1028327-66-3
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- Mulberroside A
Catalog No.:BCN6343
CAS No.:102841-42-9
- Mulberroside C
Catalog No.:BCN6344
CAS No.:102841-43-0
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
Levetiracetam in Compare to Sodium Valproate for Prophylaxis in Chronic Migraine Headache: A Randomized Double-Blind Clinical Trial.[Pubmed:28356053]
Curr Clin Pharmacol. 2017;12(1):55-59.
BACKGROUND: Migraine is not curable, but preventive treatments are usually used to decrease the intensity and frequency of headache attacks. Different therapeutic options are widely studied for chronic migraine (CM), but all of them have different inefficacies. OBJECTIVE: The aim of this study was to compare the efficacy of Levetiracetam versus sodium valproate in the treatment of CM. METHODS: A randomized controlled clinical trial was conducted on 62 patients with chronic migraine (30 patients in intervention group-treated with Levetiracetam and 32 patients in control group- treated with sodium valproate). The treatment regimen consisted of initial dose of Levetiracetam or sodium valproate 500 mg daily which increased to 500 mg two times a day after two weeks. The treatment response was evaluated by measuring pain frequency, pain severity, and the MIDAS (migraine disability assessment) score over three months follow-up. RESULTS: During a three-month follow-up, the mean of headache frequency, severity, and MIDAS score were changed significantly. The rate of decrease in headache frequency was higher in control group than intervention group ((6.7+/-2.7 and 14.4+/-5.3 day/month, respectively) (P<0.001). Also, headache severity and MIDAS score significantly decreased in the control group than intervention group (3.4+/-1.1 and 5.7+/-1.6, respectively P<0.001, 16.7 +/- 6.1 and 30.2+/-9.8, respectively (P<0.001). CONCLUSIONS: According to our findings, Levetiracetam offered improvement in headache frequency, severity, and MIDAS score in patients with CM. However, Levetiracetam was not effective enough for chronic migraine as valproate, despite some significant effect. Thus Levetiracetam can be one of the choices for limited chronic migraine subjects who are in contraindication of Valproate.
Levetiracetam Clinical Pharmacokinetic Monitoring in Pediatric Patients with Epilepsy.[Pubmed:28353057]
Clin Pharmacokinet. 2017 Nov;56(11):1267-1285.
Levetiracetam is a broad-spectrum antiepileptic drug (AED) with a unique mechanism of action. Older AEDs can cause serious short- and long-term adverse drug reactions and complications, rendering them undesirable to use in pediatric patients. Characteristics that make Levetiracetam a near-ideal AED include its broad spectrum of activity, good tolerability profile, and minimal drug-drug interactions. Clinical pharmacokinetic monitoring (CPM) is often recommended in pediatric patients for certain AEDs due to large interindividual pharmacokinetic differences and unpredictable drug disposition. Our objective was to determine whether monitoring Levetiracetam concentrations is warranted for pediatric patients with epilepsy, using a previously published 9-step decision-making algorithm. A literature search of the MEDLINE (1946-August 2016), EMBASE (1974-August 2016), CENTRAL, and Google Scholar databases was performed to identify relevant English-language articles and answer the questions posed in the algorithm for Levetiracetam CPM in pediatric epilepsies. Additional articles were identified from a manual bibliographic review of the relevant literature. We found that Levetiracetam CPM met some criteria of the algorithm: Levetiracetam is an appropriate adjunctive or monotherapy for pediatric patients with either focal or generalized seizures; it is readily measurable in plasma, with an appropriate degree of sensitivity, accuracy, and precision; it exhibits interindividual variation in pharmacokinetics; often, its pharmacologic effect cannot be easily measured; and the duration of therapy is expected to be long-term. However, important criteria not met include the following: there is no clear evidence for a concentration-response relationship for efficacy or toxicity; the proposed therapeutic range of 12-46 mug/mL is not well-defined and is generally considered as wide. Thus, clinical decision making is unlikely to be affected as a result of routine Levetiracetam CPM. In general, routine CPM of Levetiracetam cannot be recommended for pediatric patients with epilepsy. However, CPM may be beneficial in select cases, such as patients in whom noncompliance is suspected, those who have severe overdoses, those switching between product brands, or patients for whom an 'individual therapeutic concentration' is documented. Nonetheless, in the majority of pediatric patients with epilepsy, measurement of Levetiracetam concentrations is not expected to yield a therapeutic benefit. Thus, clinical assessment and judgment, without measuring drug concentrations, remain the monitoring strategy of choice for Levetiracetam therapy.
Levetiracetam synergizes with gabapentin, pregabalin, duloxetine and selected antioxidants in a mouse diabetic painful neuropathy model.[Pubmed:28332005]
Psychopharmacology (Berl). 2017 Jun;234(11):1781-1794.
RATIONALE: We have reported that Levetiracetam, a novel anticonvulsant with analgesic properties, synergizes with ibuprofen/aspirin/paracetamol in a model of diabetic painful neuropathy (DPN). Most guidelines recommend gabapentin, pregabalin, and duloxetine as first- or second-line agents for DPN. OBJECTIVE: We examined the effects of combination treatment of first-/second-line analgesics with Levetiracetam in a model of DPN. Additionally, the Levetiracetam's combinations with antioxidants, low dose of aspirin, coenzyme Q10, or alpha-lipoic acid were evaluated. METHODS: Diabetes was induced in C57BL/6 mice with a single high dose of streptozotocin. The antinociceptive effects of orally administered Levetiracetam, gabapentin, pregabalin, duloxetine (acute treatment) and aspirin, coenzyme Q10, and alpha-lipoic acid (preventive 7-day treatment), as well as combinations of Levetiracetam with individual drugs were examined in the tail-flick test. In combination experiments, the drugs were coadministered in fixed-dose fractions of single-drug ED50; the type of interaction was determined by isobolographic analysis. RESULTS: About 60-, 32-, 30-, 26-, 18-, and 6-fold reductions of doses of both drugs in Levetiracetam combinations with pregabalin, gabapentin, coenzyme Q10, aspirin, duloxetine, and alpha-lipoic acid, respectively, were detected. CONCLUSIONS: Combinations of Levetiracetam with gabapentin/pregabalin/duloxetine that target different mechanisms/sites of action involved in DPN, as well as combinations of Levetiracetam and low-dose aspirin/coenzyme Q10/alpha-lipoic acid that target underlying causes of DPN, produce marked synergistic interactions in reducing nociception in diabetic mice. This suggests that these combination treatments might be of great benefit for diabetic patients and should be explored further in clinical trials.
Brand-to-generic levetiracetam switch in patients with epilepsy in a routine clinical setting.[Pubmed:28363098]
Seizure. 2017 May;48:1-6.
PURPOSE: The therapeutic equivalence of generic and brand antiepileptic drugs, based on studies performed on healthy volunteers, has been questioned. We compare, in a routine clinical setting, brand versus generic Levetiracetam (LEV) bioequivalence in patients with epilepsy and also the clinical efficacy and tolerability of the substitution. METHODS: A prospective, open-label, non-randomized, steady-state, multiple-dose, bioequivalence study was conducted in 12 patients with epilepsy (5 females), with a mean age of 38.4+/-16.2 years. Patients treated with the brand LEV (Keppra; UCB Pharma) were closely followed for a four-week period and subsequently switched to a generic LEV (Pharmaten) and followed for another four-week period. Blood samples were collected at the end of each 4-week period, during a dose interval for each formulation, for LEV concentration measurements by liquid chromatography mass spectrometry. Steady-state area under the curve (AUC) and peak plasma concentration (Cmax) data were subjected to conventional average bioequivalence analysis. Secondary clinical outcomes, including seizure frequency and adverse events, were recorded. RESULTS: Patients had epilepsy for a mean period of 14.1+/-10.6years and the mean daily LEV dose was 2583.3+/-763.7mg. The mean AUC+/-SD and Cmax+/-SD was 288.4+/-86.3(mg/L)h and 37.8+/-10.4mg/L respectively for brand LEV and 319.2+/-104.7(mg/L)h and 41.6+/-12.3mg/L respectively for the generic LEV. Statistic analysis showed no statistical significant difference in bioequivalence. Also, no change in seizures frequency and/or adverse events was recorded. CONCLUSIONS: In our clinical setting, generic LEV was determined to be bioequivalent to brand LEV. Furthermore, seizures frequency or/and adverse events were not affected upon switching from brand to generic LEV.
Levetiracetam: the profile of a novel anticonvulsant drug-part I: preclinical data.[Pubmed:17461889]
CNS Drug Rev. 2007 Spring;13(1):43-56.
The objective of this article was to review and summarize the available reports on the preclinical profile of the novel anticonvulsant drug Levetiracetam (LEV). Therefore, a careful search was conducted in the MEDLINE database and combined with guidelines from regulatory agencies, proceedings of professional scientific meetings, and information provided by the manufacturers. This article provides detailed information on the anticonvulsant effects of LEV in various animal models of epilepsy and on its pharmacology in laboratory animals. The mechanism of action of LEV is reviewed, with special regard to its recently discovered binding site, the synaptic vesicle protein 2A. In general, LEV is shown to be a safe, broad-spectrum anticonvulsant drug with highly beneficial pharmacokinetic properties and a distinct mechanism of action. The clinical studies with LEV will be discussed in the second part of this review article to be published subsequently.
Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection.[Pubmed:17408599]
Brain Res. 2007 Jun 2;1151:55-61.
Enhancement of the glutamatergic excitatory synaptic transmission efficacy in the FeCl3 induced epilepsy model is associated with changes in the levels of glutamate and GABA transporter proteins. This study examined the effect of Levetiracetam (LEV) on glutamate overflow and glutamate/GABA transporters expression in rats with epileptogenesis induced by the amygdalar injection of 1.0 microl of 100 mM FeCl3 (epileptic rat) and in control rats receiving amygdalar acidic saline injection (non-epileptic rat). In amygdalar acidic saline injected rats, 40 mM KCl-evoked glutamate overflow was significantly suppressed by both 32 and 100 microM LEV co-perfusion. In unilateral amygdalar FeCl3 injected rats, 32 microM LEV was ineffective, but the 100 microM LEV statistically suppressed glutamate overflow. Western blotting was employed to determine the hippocampal expression of glutamate/GABA transporters in epileptic or non-epileptic rats. The rats were treated for 14 days with 54 mg/kg LEV or vehicle intraperitoneally injection. Following 14 days of treatment, the ipsilateral hippocampus was removed for a Western blot analysis. In non-epileptic rats, the expression increased for all of the glutamate and GABA transporters (GLAST, GLT-1, EAAC-1, GAT-1 and GAT-3) while the glutamate transporter regulating protein (GTRAP3-18) decreased in comparison to those of normal rats that were treated with the vehicle. In epileptic rats receiving LEV, the EAAC-1 and GAT-3 levels increased while GTRAP3-18 (89%) decreased in comparison to those of the epileptic rats treated with the vehicle. GTRAP3-18 inhibitor regulates glutamate-binding affinity to EAAC-1. The anti-epileptic action of LEV may be partially due to a reduction of glutamate-induced excitotoxicity and an enhancement of the GABAergic inhibition as observed with the inhibitory effect on the 40 mM KCl-evoked glutamate overflow. These conclusions are supported by the increase in the expression of glial glutamate transporters (GLAST and GLT-1), and the increase in the expression of EAAC-1 and GAT-3 associated with a decrease in GTRAP3-18. The increased expression of EAAC-1 and the decreased expression of GTRAP3-18 in association with the up-regulation of GAT-3 due to such continual LEV administration was thus found to enhance GABA synthesis and reverse the transport of GABA both in non-epileptic and epileptic rats. The suppression of glutamate excitation and the enhancement of GABA inhibition in the rats with continual LEV administration is a result of the up-regulation of glutamate and GABA transporters with the down-regulation of GTRAP3-18. These observations together demonstrated the critical molecular mechanism of the anti-epileptic activity of LEV.


