Lacinilene CCAS# 41653-72-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41653-72-9 | SDF | Download SDF |
| PubChem ID | 170551 | Appearance | Yellow powder |
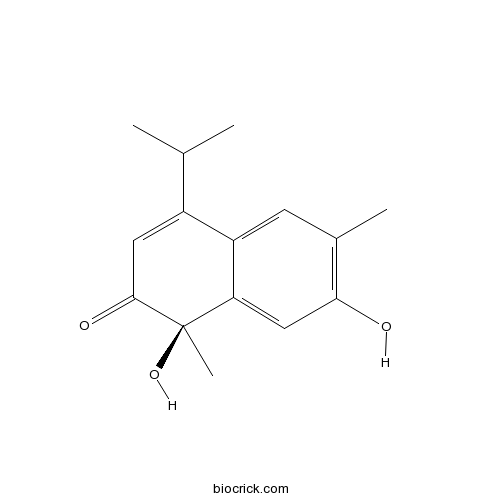
| Formula | C15H18O3 | M.Wt | 246.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R)-1,7-dihydroxy-1,6-dimethyl-4-propan-2-ylnaphthalen-2-one | ||
| SMILES | CC1=C(C=C2C(=C1)C(=CC(=O)C2(C)O)C(C)C)O | ||
| Standard InChIKey | JLCJSBOHWRDWQW-OAHLLOKOSA-N | ||
| Standard InChI | InChI=1S/C15H18O3/c1-8(2)10-6-14(17)15(4,18)12-7-13(16)9(3)5-11(10)12/h5-8,16,18H,1-4H3/t15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lacinilene C methyl ether elicits contraction of TSM by enhancing the movement of calcium through potential-dependent channels of smooth muscle cell membranes. 2. Lacinilene C, lacinilene C 7-methyl ether, scopoletin, 2-hydroxy-7-methoxycadalene and 2,7-dihydroxycadalene are 5 induced phytoalexins in cotton leaves. |
| Targets | P450 (e.g. CYP17) | 5-HT Receptor | Histamine Receptor |

Lacinilene C Dilution Calculator

Lacinilene C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ciclopirox ethanolamine
Catalog No.:BCC4372
CAS No.:41621-49-2
- Ylangenol
Catalog No.:BCN6705
CAS No.:41610-69-9
- (-)-Pinoresinol 4-O-glucoside
Catalog No.:BCN7251
CAS No.:41607-20-9
- 4,R-ajmalicine N-oxide
Catalog No.:BCN5473
CAS No.:41590-29-8
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- Poricoic acid G
Catalog No.:BCN8267
CAS No.:415724-84-4
- RI-1
Catalog No.:BCC1896
CAS No.:415713-60-9
- SN-6
Catalog No.:BCC7273
CAS No.:415697-08-4
- N6-Cyclopentyladenosine
Catalog No.:BCC7160
CAS No.:41552-82-3
- 1-(3,4-dimethoxyphenyl)-2-(4-allly-2,6-dimethoxyphenoxy)propan-1-ol
Catalog No.:BCN1445
CAS No.:41535-95-9
- 2,6,16-Kauranetriol
Catalog No.:BCN5472
CAS No.:41530-90-9
- Koaburaside monomethyl ether
Catalog No.:BCN5471
CAS No.:41514-64-1
- Koaburaside
Catalog No.:BCN5475
CAS No.:41653-73-0
- 7-O-Methyl morroniside
Catalog No.:BCN3882
CAS No.:41679-97-4
- Catharanthine hemitartrate
Catalog No.:BCN8463
CAS No.:4168-17-6
- 2H-1-Benzopyran-7-yloxy
Catalog No.:BCN3580
CAS No.:41680-08-4
- 4',7-Dihydroxyflavanone
Catalog No.:BCC8333
CAS No.:41680-09-5
- 8-Methyleugenitol
Catalog No.:BCN6459
CAS No.:41682-21-7
- Arctigenin 4'-O-beta-gentiobioside
Catalog No.:BCN2847
CAS No.:41682-24-0
- 8-Acetoxypentadeca-1,9Z-diene-4,6-diyn-3-ol
Catalog No.:BCN1444
CAS No.:41682-30-8
- Epiaschantin
Catalog No.:BCN7206
CAS No.:41689-50-3
- Epimagnolin A
Catalog No.:BCN7831
CAS No.:41689-51-4
- Indicine N-oxide
Catalog No.:BCN1996
CAS No.:41708-76-3
- Croalbidine
Catalog No.:BCN2068
CAS No.:41714-30-1
RNAi construct of a cytochrome P450 gene CYP82D109 blocks an early step in the biosynthesis of hemigossypolone and gossypol in transgenic cotton plants.[Pubmed:25794893]
Phytochemistry. 2015 Jul;115:59-69.
Naturally occurring terpenoid aldehydes from cotton, such as hemigossypol, gossypol, hemigossypolone, and the heliocides, are important components of disease and herbivory resistance in cotton. These terpenoids are predominantly found in the glands. Differential screening identified a cytochrome P450 cDNA clone (CYP82D109) from a Gossypium hirsutum cultivar that hybridized to mRNA from glanded cotton but not glandless cotton. Both the D genome cotton Gossypium raimondii and A genome cotton Gossypium arboreum possessed three additional paralogs of the gene. G. hirsutum was transformed with a RNAi construct specific to this gene family and eight transgenic plants were generated stemming from at least five independent transformation events. HPLC analysis showed that RNAi plants, when compared to wild-type Coker 312 (WT) plants, had a 90% reduction in hemigossypolone and heliocides levels, and a 70% reduction in gossypol levels in the terminal leaves, respectively. Analysis of volatile terpenes by GC-MS established presence of an additional terpene (MW: 218) from the RNAi leaf extracts. The (1)H and (13)C NMR spectroscopic analyses showed this compound was delta-cadinen-2-one. Double bond rearrangement of this compound gives 7-hydroxycalamenene, a Lacinilene C pathway intermediate. delta-Cadinen-2-one could be derived from delta-cadinene via a yet to be identified intermediate, delta-cadinen-2-ol. The RNAi construct of CYP82D109 blocks the synthesis of desoxyhemigossypol and increases the induction of Lacinilene C pathway, showing that these pathways are interconnected. Lacinilene C precursors are not constitutively expressed in cotton leaves, and blocking the gossypol pathway by the RNAi construct resulted in a greater induction of the Lacinilene C pathway compounds when challenged by pathogens.
Lacinilene C methyl ether (LCME) constricts tracheal smooth muscle.[Pubmed:3338430]
Environ Res. 1988 Feb;45(1):118-26.
Lacinilene C methyl ether (LCME) is a constituent of the cotton plant and has been implicated as a causative agent of byssinosis. The effect of synthetic LCME on the behavior of isolated strips of canine tracheal smooth muscle (TSM) was examined in tissue bath experiments. LCME (0.64-6.6 x 10(-4) M) caused slowly developing but strong and sustained contractions of TSM strips. Blockade of acetycholine, 5-hydroxytryptamine (5-HT), and histamine H1 receptors with atropine, methysergide, and pyrilamine, respectively, had no effect on constrictions induced by LCME. In contrast, the calcium channel antagonist, verapamil, significantly reduced the response to LCME. Moreover, addition of the beta receptor agonist, isoproterenol, at the peak of LCME-induced contractions relaxed tissues in a concentration-dependent manner. We conclude that LCME elicits contraction of TSM by enhancing the movement of calcium through potential-dependent channels of smooth muscle cell membranes. This mechanism of action does not require activation of specific membrane receptors for acetylcholine, 5-HT, and histamine.


