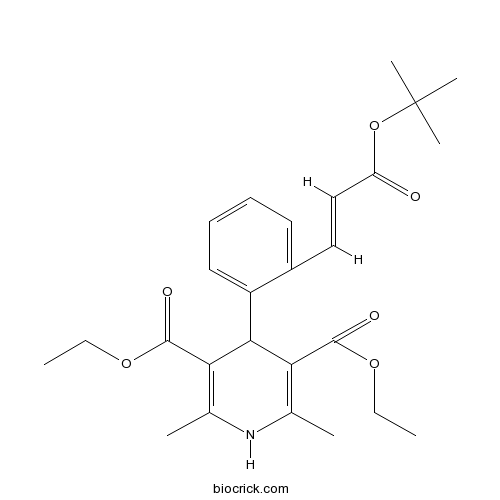
LacidipineL-type calcium channel blocker CAS# 103890-78-4 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 103890-78-4 | SDF | Download SDF |
| PubChem ID | 5311217 | Appearance | Powder |
| Formula | C26H33NO6 | M.Wt | 455.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | diethyl 2,6-dimethyl-4-[2-[(E)-3-[(2-methylpropan-2-yl)oxy]-3-oxoprop-1-enyl]phenyl]-1,4-dihydropyridine-3,5-dicarboxylate | ||
| SMILES | CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2C=CC(=O)OC(C)(C)C)C(=O)OCC)C)C | ||
| Standard InChIKey | GKQPCPXONLDCMU-CCEZHUSRSA-N | ||
| Standard InChI | InChI=1S/C26H33NO6/c1-8-31-24(29)21-16(3)27-17(4)22(25(30)32-9-2)23(21)19-13-11-10-12-18(19)14-15-20(28)33-26(5,6)7/h10-15,23,27H,8-9H2,1-7H3/b15-14+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lacidipine Dilution Calculator

Lacidipine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1952 mL | 10.976 mL | 21.952 mL | 43.9039 mL | 54.8799 mL |
| 5 mM | 0.439 mL | 2.1952 mL | 4.3904 mL | 8.7808 mL | 10.976 mL |
| 10 mM | 0.2195 mL | 1.0976 mL | 2.1952 mL | 4.3904 mL | 5.488 mL |
| 50 mM | 0.0439 mL | 0.2195 mL | 0.439 mL | 0.8781 mL | 1.0976 mL |
| 100 mM | 0.022 mL | 0.1098 mL | 0.2195 mL | 0.439 mL | 0.5488 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lacidipine (Lacipil, Motens) is a L-type calcium channel blocker.Lacidipine, a novel third-generation dihydropyridine calcium channel blocker, has been demonstrated effective for hypertension. lacidipine protects HKCs against apoptosis induced by ATP depl
- Procyanidin A1
Catalog No.:BCN6809
CAS No.:103883-03-0
- Cycloart-25-ene-3,24-diol
Catalog No.:BCN5852
CAS No.:10388-48-4
- Lazabemide hydrochloride
Catalog No.:BCC7371
CAS No.:103878-83-7
- 4'-O-Methyllicoflavanone
Catalog No.:BCN4827
CAS No.:1038753-13-7
- 7-Prenylumbelliferone
Catalog No.:BCN2938
CAS No.:10387-50-5
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- MBX-2982
Catalog No.:BCC1732
CAS No.:1037792-44-1
- Ganoderic acid C2
Catalog No.:BCN3036
CAS No.:103773-62-2
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- MK-4827 hydrochloride
Catalog No.:BCC4173
CAS No.:1038915-64-8
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
- Mannioside A
Catalog No.:BCN5853
CAS No.:1038922-95-0
- 17β-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one
Catalog No.:BCC8444
CAS No.:1039-17-4
- Maxacalcitol
Catalog No.:BCC1730
CAS No.:103909-75-7
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
Preparation and in vitro/in vivo Evaluation of Lacidipine by Adsorption onto Fumed Silica Using Supercritical Carbon Dioxide.[Pubmed:26634790]
Curr Drug Deliv. 2016;13(7):1053-1064.
The aim of this study was to design a silica-supported solid dispersion of Lacidipine (LCDP) to enhance the dissolution rate and oral absorption using supercritical CO2 (scCO2) as a solvent. The formulation was characterized using differential scanning calorimetry, powder X-ray diffraction, scanning electron microscopy and fourier transformed infrared spectroscopy. In the dissolution test, LCDP-scCO2 formulation showed a significantly enhanced dissolution compared with LCDPsilica physical mixture and a faster dissolution rate than Lacipil(R) under different dissolution conditions. In an in vivo test, the area under concentration-time curve and Cmax of LCDP-scCO2 formulation was 9.23 and 23.78 fold greater than LCDP-silica physical mixture (1:15, w/w), respectively, whereas the corresponding values were 1.92 and 2.80 fold greater than Lacipil(R), respectively. Our results showed that the solid dispersion prepared by supercritical fluids technology is a feasible method to enhance the oral bioavailability of LCDP.
Enhanced Solubility and Dissolution Rate of Lacidipine Nanosuspension: Formulation Via Antisolvent Sonoprecipitation Technique and Optimization Using Box-Behnken Design.[Pubmed:27506564]
AAPS PharmSciTech. 2017 May;18(4):983-996.
Lacidipine (LCDP) is a highly lipophilic calcium channel blocker of poor aqueous solubility leading to poor oral absorption. This study aims to prepare and optimize LCDP nanosuspensions using antisolvent sonoprecipitation technique to enhance the solubility and dissolution of LCDP. A three-factor, three-level Box-Behnken design was employed to optimize the formulation variables to obtain LCDP nanosuspension of small and uniform particle size. Formulation variables were as follows: stabilizer to drug ratio (A), sodium deoxycholate percentage (B), and sonication time (C). LCDP nanosuspensions were assessed for particle size, zeta potential, and polydispersity index. The formula with the highest desirability (0.969) was chosen as the optimized formula. The values of the formulation variables (A, B, and C) in the optimized nanosuspension were 1.5, 100%, and 8 min, respectively. Optimal LCDP nanosuspension had particle size (PS) of 273.21 nm, zeta potential (ZP) of -32.68 mV and polydispersity index (PDI) of 0.098. LCDP nanosuspension was characterized using x-ray powder diffraction, differential scanning calorimetry, and transmission electron microscopy. LCDP nanosuspension showed saturation solubility 70 times that of raw LCDP in addition to significantly enhanced dissolution rate due to particle size reduction and decreased crystallinity. These results suggest that the optimized LCDP nanosuspension could be promising to improve oral absorption of LCDP.
Solvent-shift strategy to identify suitable polymers to inhibit humidity-induced solid-state crystallization of lacidipine amorphous solid dispersions.[Pubmed:26869398]
Int J Pharm. 2016 Apr 30;503(1-2):238-46.
The solvent-shift strategy was used to identify appropriate polymers that inhibit humidity-induced solid-state crystallization of amorphous solid dispersions (ASDs). Lacidipine with the polymers, PVP-K30, HPMC-E5 or Soluplus, were combined to form amorphous solid dispersions prepared by solvent evaporation. The formulations were characterized by differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier-transform infrared spectroscopy (FT-IR) and were subjected to in vitro dissolution testing. The moisture had a significant impact on the amount dissolved for the solid dispersions. Molecular docking studies established that hydrogen bonding was critical for the stabilization of the solid dispersions. The rank order of the binding energy of the drug-polymer association was Soluplus (-6.21 kcal/mol)>HPMC-E5 (-3.21 kcal/mol)>PVP-K30 (-2.31 kcal/mol). PVP-K30 had the highest water uptake among the polymers, as did ASD system of Lacidipine-PVP-K30 ASDs. In the Soluplus ASDs, with its strong drug-polymer interactions and low water uptake, moisture-induced solid-state crystallization was not observed.
Novel non-ionic surfactant proniosomes for transdermal delivery of lacidipine: optimization using 2(3) factorial design and in vivo evaluation in rabbits.[Pubmed:26758033]
Drug Deliv. 2016 Jun;23(5):1608-22.
CONTEXT: Proniosomes offer a versatile vesicle drug delivery concept with potential for delivery of drugs via transdermal route. OBJECTIVES: To develop proniosomal gel using cremophor RH 40 as non-ionic surfactant containing the antihypertensive drug Lacidipine for transdermal delivery so as to avoid its extensive first pass metabolism and to improve its permeation through the skin. MATERIALS AND METHODS: Proniosomes containing 1% Lacidipine were prepared by the coacervation phase separation method, characterized, and optimized using a 2(3) full factorial design to define the optimum conditions to produce proniosomes with high entrapment efficiency, minimal vesicle size, and high-percentage release efficiency. The amount of cholesterol (X1), the amount of soya lecithin (X2), and the amount of cremophor RH 40 (X3) were selected as three independent variables. RESULTS AND DISCUSSION: The system F4 was found to fulfill the maximum requisite of an optimum system because it had minimum vesicle size, maximum EE, maximum release efficiency, and maximum desirability. The optimized system (F4) was then converted to proniosomal gel using carbopol 940 (1% w/w). In vitro permeation through excised rabbit skin study revealed higher flux (6.48 +/- 0.45) for Lacidipine from the optimized proniosomal gel when compared with the corresponding emulgel (3.04 +/- 0.13) mg/cm(2)/h. The optimized formulation was evaluated for its bioavailability compared with commercial product. Statistical analysis revealed significant increase in AUC (0 - alpha) 464.17 +/- 113.15 ng h/ml compared with 209.02 +/- 47.35 ng h/ml for commercial tablet. Skin irritancy and histopathological investigation of rat skin revealed its safety. CONCLUSIONS: Cremophor RH 40 proniosomal gel could be considered as very promising nanocarriers for transdermal delivery of Lacidipine.


