L-Quisqualic acidVery potent group I mGlu agonist CAS# 52809-07-1 |
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52809-07-1 | SDF | Download SDF |
| PubChem ID | 40539 | Appearance | Powder |
| Formula | C5H7N3O5 | M.Wt | 189.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH and to 10 mM in water | ||
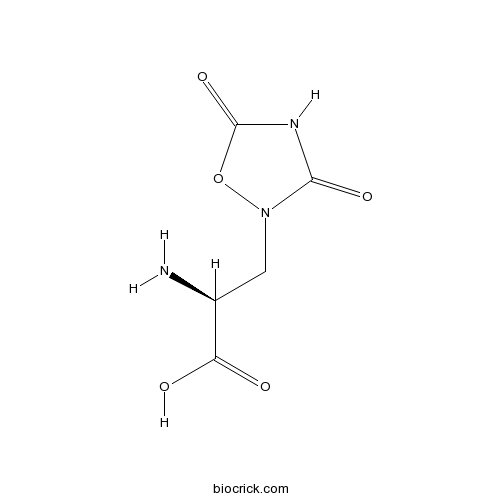
| Chemical Name | (2S)-2-amino-3-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl)propanoic acid | ||
| SMILES | C(C(C(=O)O)N)N1C(=O)NC(=O)O1 | ||
| Standard InChIKey | ASNFTDCKZKHJSW-REOHCLBHSA-N | ||
| Standard InChI | InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12)/t2-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glutamate receptor agonist acting at AMPA receptors and metabotropic glutamate receptors positively linked to phosphoinositide hydrolysis. Sensitizes neurons in hippocampus to depolarization by L-AP6 (the so called 'quis' effect). Also available as part of the Group I mGlu Receptor. |

L-Quisqualic acid Dilution Calculator

L-Quisqualic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2874 mL | 26.4368 mL | 52.8737 mL | 105.7474 mL | 132.1842 mL |
| 5 mM | 1.0575 mL | 5.2874 mL | 10.5747 mL | 21.1495 mL | 26.4368 mL |
| 10 mM | 0.5287 mL | 2.6437 mL | 5.2874 mL | 10.5747 mL | 13.2184 mL |
| 50 mM | 0.1057 mL | 0.5287 mL | 1.0575 mL | 2.1149 mL | 2.6437 mL |
| 100 mM | 0.0529 mL | 0.2644 mL | 0.5287 mL | 1.0575 mL | 1.3218 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isobavachromene
Catalog No.:BCN3192
CAS No.:52801-22-6
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
- Cyanidin Chloride
Catalog No.:BCN1231
CAS No.:528-58-5
- Delphinidin chloride
Catalog No.:BCN3015
CAS No.:528-53-0
- Fisetin
Catalog No.:BCN5024
CAS No.:528-48-3
- Magnolol
Catalog No.:BCN5687
CAS No.:528-43-8
- H-Phe(3,4-DiCl)-OH
Catalog No.:BCC3178
CAS No.:52794-99-7
- H-D-Phe(3,4-DiCl)-OH
Catalog No.:BCC3179
CAS No.:52794-98-6
- PHA 568487
Catalog No.:BCC7574
CAS No.:527680-57-5
- Medicarpin 3-O-glucoside
Catalog No.:BCN7773
CAS No.:52766-70-8
- beta-Asarone
Catalog No.:BCN5685
CAS No.:5273-86-9
- Isoelemicin
Catalog No.:BCN4760
CAS No.:5273-85-8
- Dalbergiphenol
Catalog No.:BCN7451
CAS No.:52811-31-1
- Cearoin
Catalog No.:BCN7772
CAS No.:52811-37-7
- Olean-12-ene-3,11-diol
Catalog No.:BCN5686
CAS No.:5282-14-4
- 20-Hydroxyecdysone
Catalog No.:BCN5688
CAS No.:5289-74-7
- Chamazulene
Catalog No.:BCC8145
CAS No.:529-05-5
- Ombuin
Catalog No.:BCN5691
CAS No.:529-40-8
- Myricetin
Catalog No.:BCN5692
CAS No.:529-44-2
- Gentisein
Catalog No.:BCN3356
CAS No.:529-49-7
- Azaleatin
Catalog No.:BCN8207
CAS No.:529-51-1
- Scutellarein
Catalog No.:BCN5380
CAS No.:529-53-3
- Prunin
Catalog No.:BCN5693
CAS No.:529-55-5
- Genistin
Catalog No.:BCN2396
CAS No.:529-59-9
Quisqualic acid induced sensitization and the active uptake of L-quisqualic acid by hippocampal slices.[Pubmed:8096790]
Brain Res. 1993 Mar 5;605(1):85-92.
Hippocampal CA1 pyramidal cell neurons are sensitized to depolarization by L-2-amino-4-phosphonobutanoic acid (L-AP4) following exposure to L-Quisqualic acid (QUIS). It has been proposed that induction of this 'QUIS-effect' involves uptake of L-QUIS by hippocampal cells. We have used o-phthaldialdehyde (OPA) derivatization and high performance liquid chromatographic (HPLC) separation of extracts from hippocampal slices which have been exposed to varied concentrations of L-QUIS to investigate L-QUIS uptake into hippocampal slices. We observe uptake rates such that the internal concentration of L-QUIS exceeds the bath concentration within 7 min. The fact that this uptake is concentrative indicates that it is mediated by an active transport system. In addition, uptake of L-QUIS may be linked to the induction of the QUIS-effect. At low concentrations of L-QUIS (< 4 microM), the QUIS-effect is only partially induced within the 4 min incubation time which maximally induces the effect when 16 microM L-QUIS is used. However, repeated 4 min exposure periods of slices to low L-QUIS concentrations will eventually induce the QUIS-effect even when each exposure is separated by extensive washout periods. Hence induction is dependent on both concentration and total exposure time. We also examined the effects of L-alpha-aminoadipic acid and L-serine-O-sulfate on the rate of L-QUIS uptake. Exposure of slices to these compounds prior to treatment with L-QUIS will block the physiological effects of L-QUIS. We found that these 'pre-blocking' compounds did not decrease the rate of L-QUIS uptake.(ABSTRACT TRUNCATED AT 250 WORDS)
A novel binding assay for metabotropic glutamate receptors using [3H] L-quisqualic acid and recombinant receptors.[Pubmed:12064739]
Z Naturforsch C. 2002 Mar-Apr;57(3-4):348-55.
We established a methodology to analyze radioligand binding to the recombinant type la metabotropic glutamate receptor (mGluRla). A full-length cDNA encoding mGluR1a, which was isolated from a lambda gt 11 cDNA library of human cerebellar origin, was expressed in a baculovirus/Sf9 insect cell system. Membrane fractions with recombinant receptor expression were analyzed for the binding of [3H]L-Quisqualic acid (L-QA), which is known to be a potent agonist of mGluRla. Efficient binding of the radioligand to the human receptor was observed in a saturable manner, giving an apparent Kd= 0.091 microM. [3H]L-QA bound to the human mGluR1a was displaced by known ligands such as L-QA, L-Glu, t-ACPD ((+/-)-1-aminocyclopentane-trans-1,3-dicarboxylic acid) with IC50s = 0.056, 0.97 and 4.0 microM, respectively. MCPG (alpha-methyl-4-carboxyphenylglycine) displaced the radioligand binding with lower potency. Using this binding protocol, we then evaluated the ligand ability of synthetic dipeptides. Among peptides tested, only Glu-containing dipeptides inhibited the radioligand binding, e.g. IC50 of L-Met-L-Glu was 4.3 microM. When phosphatidyl inositol turnover was assayed in mGluR1a-expressing CHO cells, L-Met-L-Glu was partially agonistic. We further expanded this [3H]L-QA binding protocol to type 5a mGluR, another member of group I mGluRs, as well as to AMPA receptor, a member of ionotropic glutamate receptors, since L-QA is also known to be a potent ligand for these receptors. Data shown here will provide a novel system not only to search for ligands for the glutamate receptors, but also to biochemically analyze the interaction modes between glutamate receptors and their ligands.
L-Quisqualic acid transport into hippocampal neurons by a cystine-sensitive carrier is required for the induction of quisqualate sensitization.[Pubmed:11566501]
Neuroscience. 2001;106(2):287-301.
A brief exposure of hippocampal slices to L-Quisqualic acid sensitizes CA1 pyramidal neurons 30-250-fold to depolarization by two classes of excitatory amino acid analogues: (1) those whose depolarizing effects are rapidly terminated following washout, e.g. L-2-amino-4-phosphonobutanoic acid (L-AP4) and L-2-amino-6-phosphonohexanoic acid (L-AP6) and (2) those whose depolarizing effects persist following washout, e.g. L-aspartate-beta-hydroxamate (L-AbetaH). This process has been termed quisqualate sensitization. In this study we directly examine the role of amino acid transport systems in the induction of quisqualate sensitization. We report that L-quisqualate is a low-affinity substrate (K(M)=0.54 mM) for a high capacity (V(max)=0.9 nmol (mg protein)(-1) min(-1)) Na(+)-dependent transport system(s) and a high-affinity substrate (K(M)=0.033 mM) for a low-capacity (V(max)=0.051 nmol (mg protein)(-1) min(-1)) transporter with properties similar to the cystine/glutamate exchange carrier, System x(c-). We present evidence that suggests that System x(c-) participates in quisqualate sensitization. First, simultaneous application of L-quisqualate and inhibitors of System x(c-), but not inhibitors of Na(+)-dependent glutamate transporters, prevents the subsequent sensitization of hippocampal neurons to phosphonates or L-AbetaH. Second, L-Quisqualic acid only sensitizes hippocampal neurons to other substrates of System x(c-), including cystine. Third, immunocytochemical analysis of L-quisqualate uptake demonstrates that only inhibitors of System x(c-) inhibit the highly concentrative uptake of L-quisqualate into a widely dispersed group of GABAergic hippocampal interneurons. We conclude that quisqualate sensitization is a direct consequence of the unique interaction of various excitatory amino acids, namely L-quisqualate, cystine, and phosphonates, with the exchange carrier, System x(c-). Therefore, the results of this study have important implications for the mechanism by which L-quisqualate, and other substrates of this transporter which are also excitatory amino acid agonists (such as glutamate and beta-N-oxalyl-L-alpha,beta-diaminopropionic acid, beta-L-ODAP) may trigger neurotoxicity.
Effects of quisqualic acid analogs on metabotropic glutamate receptors coupled to phosphoinositide hydrolysis in rat hippocampus.[Pubmed:8532164]
Neuropharmacology. 1995 Aug;34(8):829-41.
L-Glutamic acid (L-Glu) and L-aspartic acid (L-Asp) activate several receptor subtypes, including metabotropic Glu receptors coupled to phosphoinositide (PI) hydrolysis. Quisqualic acid (Quis) is the most potent agonist of these receptors. There is evidence that activation of these receptors may cause a long lasting sensitization of neurons to depolarization, a phenomenon called the Quis effect. The purpose of the current studies was to use Quis analogs and the recently identified metabotropic receptor antagonist, (+)-alpha-methyl-4-carboxy-phenylglycine((+)-MCPG), to define the structural properties required for interaction with the metabotropic receptors coupled to PI hydrolysis and to determine if the Quis effect is mediated by these receptors. The effects of Quis analogs on PI hydrolysis were studied in the absence or presence of the metabotropic receptor-specific agonist 1SR,3RS-1-amino-1,3-cyclopentanedicarboxylic acid (1SR,3RS-ACPD) in neonatal rat hippocampus. Some of the compounds that induce the Quis effect also stimulate PI hydrolysis, including Quis itself and 9 (homoquisqualic acid). Not all of the Quis analogs that stimulate PI hydrolysis, however, induce the Quis effect, including 7A (EC50 = 750 +/- 150 microM) and (RS)-4-bromohomoibotenic acid (BrHI) (EC50 = 130 +/- 40 microM). Although (+)-MCPG blocked PI hydrolysis stimulated by Quis (IC50 = 370 +/- 70 microM), it had no effect on the induction of the Quis effect. Other Quis analogs did not stimulate PI hydrolysis but rather blocked the effects of 1SR,3RS-ACPD. The IC50 values were 240 +/- 70 microM for 2, 250 +/- 90 microM for 3, and 640 +/- 200 microM for 4. Data for inhibition by 2 and 3 were consistent with non-competitive mechanisms of action. These studies provide new information about the structural features of Quis required for interaction with metabotropic receptors coupled to PI hydrolysis and provide evidence that the Quis effect is not mediated by (+)-MCPG sensitive subtypes of these receptors.
Utilization of the resolved L-isomer of 2-amino-6-phosphonohexanoic acid (L-AP6) as a selective agonist for a quisqualate-sensitized site in hippocampal CA1 pyramidal neurons.[Pubmed:7953634]
Brain Res. 1994 Jun 27;649(1-2):203-7.
Brief exposure of rat hippocampal slices to quisqualic acid (QUIS) sensitizes neurons to depolarization by the alpha-amino-omega-phosphonate excitatory amino acid (EAA) analogues AP4, AP5 and AP6. These phosphonates interact with a novel QUIS-sensitized site. Whereas L-AP4 and D-AP5 cross-react with other EAA receptors, DL-AP6 has been shown to be relatively selective for the QUIS-sensitized site. This specificity of DL-AP6, in conjunction with the apparent preference of this site for L-isomers, suggested that the hitherto unavailable L-isomer of AP6 would be a potent and specific agonist. We report the resolution of the D- and L-enantiomers of AP6 by fractional crystallization of the L-lysine salt of DL-AP6. We also report the pharmacological responses of kainate/AMPA, NMDA, lateral perforant path L-AP4 receptors and the CA1 QUIS-sensitized site to D- and L-AP6, and compare these responses to the D- and L-isomers of AP3, AP4, AP5 and AP7. The D-isomers of AP4, AP5 and AP6 were 5-, 3- and 14-fold less potent for the QUIS-sensitized site than their respective L-isomers. While L-AP4 and L-AP5 cross-reacted with NMDA and L-AP4 receptors, L-AP6 was found to be highly potent and specific for the QUIS-sensitized site (IC50 = 40 microM). Its IC50 values for kainate/AMPA, NMDA and L-AP4 receptors were > 10, 3 and 0.8 mM, respectively. As with AP4 and AP5, sensitization to L-AP6 was reversed by L-alpha-aminoadipate.
(S)-homoquisqualate: a potent agonist at the glutamate metabotropic receptor.[Pubmed:1324071]
Br J Pharmacol. 1992 Jul;106(3):509-10.
The synthetic quisqualate analogue, (S)-homoquisqualate was examined for activity at the glutamate metabotropic receptor, in relation to its ability to stimulate phosphoinositide hydrolysis in rat pup cerebro-cortical slices. The compound produced a robust increase in hydrolysis (EC50 = 50.2 +/- 1.6 microM), which, in common with responses to quisqualate and (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate ((1S,3R)-ACPD), was antagonized uncompetitively by L-2-amino-3-phosphonopropionate (L-AP3). In contrast to quisqualate which exhibits low efficacy, (S)-homoquisqualate behaves as a full agonist at the metabotropic receptor.


