Kaempferol tetraacetateCAS# 16274-11-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16274-11-6 | SDF | Download SDF |
| PubChem ID | 14130926 | Appearance | Powder |
| Formula | C23H18O10 | M.Wt | 454.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
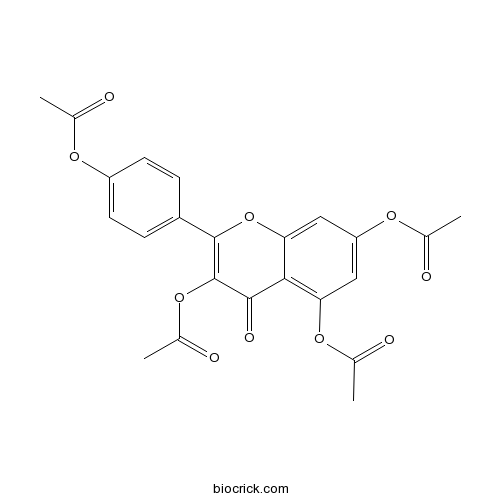
| Chemical Name | [4-(3,5,7-triacetyloxy-4-oxochromen-2-yl)phenyl] acetate | ||
| SMILES | CC(=O)OC1=CC=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)OC(=O)C)OC(=O)C)OC(=O)C | ||
| Standard InChIKey | UXAYHERJWMTSFV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H18O10/c1-11(24)29-16-7-5-15(6-8-16)22-23(32-14(4)27)21(28)20-18(31-13(3)26)9-17(30-12(2)25)10-19(20)33-22/h5-10H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kaempferol tetraacetate exhibits significant cytotoxicity in vitro against three human cell lines HL-60, U937 and SK-MEL-1. 2. Kaempferol tetraacetate is a potent antiplatelet agent. |
| Targets | Antifection |

Kaempferol tetraacetate Dilution Calculator

Kaempferol tetraacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2007 mL | 11.0035 mL | 22.007 mL | 44.0141 mL | 55.0176 mL |
| 5 mM | 0.4401 mL | 2.2007 mL | 4.4014 mL | 8.8028 mL | 11.0035 mL |
| 10 mM | 0.2201 mL | 1.1004 mL | 2.2007 mL | 4.4014 mL | 5.5018 mL |
| 50 mM | 0.044 mL | 0.2201 mL | 0.4401 mL | 0.8803 mL | 1.1004 mL |
| 100 mM | 0.022 mL | 0.11 mL | 0.2201 mL | 0.4401 mL | 0.5502 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Deoxyjacareubin
Catalog No.:BCN6573
CAS No.:16265-56-8
- HQL 79
Catalog No.:BCC7703
CAS No.:162641-16-9
- AT 56
Catalog No.:BCC6036
CAS No.:162640-98-4
- AZD3759
Catalog No.:BCC6475
CAS No.:1626387-80-1
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
- Sorokinianin
Catalog No.:BCN6978
CAS No.:162616-73-1
- Eriosemation
Catalog No.:BCN3738
CAS No.:162616-72-0
- Isolupalbigenin
Catalog No.:BCN6835
CAS No.:162616-70-8
- 3-Hydroxy-5,7-dimethoxy-3',4'-methylenedioxyflavan
Catalog No.:BCN1540
CAS No.:162602-04-2
- Broussoflavonol F
Catalog No.:BCN3571
CAS No.:162558-94-3
- Fmoc-Dap(Boc)-OH
Catalog No.:BCC3188
CAS No.:162558-25-0
- CDP 840 hydrochloride
Catalog No.:BCC7814
CAS No.:162542-90-7
- 3,4-Dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran
Catalog No.:BCN1539
CAS No.:16274-33-2
- 1-Hydroxy-2-prenylnaphthalene
Catalog No.:BCN1722
CAS No.:16274-34-3
- AZD8186
Catalog No.:BCC6470
CAS No.:1627494-13-6
- LDN-214117
Catalog No.:BCC5528
CAS No.:1627503-67-6
- Anemarrhena B
Catalog No.:BCN7592
CAS No.:1627521-95-2
- WAY-100635
Catalog No.:BCC2053
CAS No.:162760-96-5
- Yunnancoronarin A
Catalog No.:BCN1723
CAS No.:162762-93-8
- PFI-2
Catalog No.:BCC5561
CAS No.:1627676-59-8
- LJI308
Catalog No.:BCC6538
CAS No.:1627709-94-7
- 7-Epi-10-oxo-docetaxel
Catalog No.:BCC5410
CAS No.:162784-72-7
- IEM 1754 dihydrobroMide
Catalog No.:BCC5049
CAS No.:162831-31-4
- 2,3,9,10-Tetrahydroxyberberine
Catalog No.:BCN3550
CAS No.:162854-37-7
Selective methylation of kaempferol via benzylation and deacetylation of kaempferol acetates.[Pubmed:25815082]
Beilstein J Org Chem. 2015 Feb 25;11:288-93.
A strategy for selective mono-, di- and tri-O-methylation of kaempferol, predominantly on the basis of selective benzylation and controllable deacetylation of kaempferol acetates, was developed. From the selective deacetylation and benzylation of Kaempferol tetraacetate (1), 3,4',5,-tri-O-acetylkaempferol (2) and 7-O-benzyl-3,4'5,-tri-O-acetylkaempferol (8) were obtained, respectively. By controllable deacetylation and followed selective or direct methylation of these two intermediates, eight O-methylated kaempferols were prepared with 51-77% total yields from kaempferol.
Cytotoxic activities of flavonoid glycoside acetates from Consolida oliveriana.[Pubmed:18214815]
Planta Med. 2008 Feb;74(2):171-4.
The flavonoids kaempferol, quercetin, trifolin, hyperoside 2''- and 6''- acetates, 7-glucotrifolin, biorobin and robinin were isolated from the aerial parts of Consolida oliveriana. Their derivatives Kaempferol tetraacetate, quercetin pentaacetate, trifolin heptaacetate and hyperoside octaacetate exhibited significant cytotoxicity IN VITRO against three human cell lines HL-60, U937 and SK-MEL-1 while hyperoside 2''-acetate, hyperoside-6''-acetate, glucotrifolin decaacetate and heptamethyltrifolin were inactive.
Antiplatelet effects and vasorelaxing action of some constituents of Formosan plants.[Pubmed:8350094]
J Nat Prod. 1993 Jun;56(6):929-34.
Various xanthones as well as quercetin have been shown to exhibit antiplatelet activity. A series of anthraquinones analogues structurally related to xanthones and a series of quercetin-related compounds were tested for their antiplatelet effects. Emodin, frangulin B, Kaempferol tetraacetate, quercetin-3-O-galactoside octaacetate, rhamnazin triacetate, and rhamnetin tetraacetate were found to be potent antiplatelet agents, and 1,8-dihydroxy-6-methoxy-3-methylanthraquinone 8-O-rhamnosyl-(1-->2)-glucoside, rhamnustrioside undecaacetate, rutin decaacetate, and quercetin-3-O-(6-O-alpha-L-arabinopyranosyl)-beta-D-galactopyranos ide decaacetate showed significant antiplatelet effects. Quercetin showed vasorelaxing action in rat thoracic aorta.


