K858Selective ATP-uncompetitive mitotic kinesin Eg5 inhibitor CAS# 72926-24-0 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 72926-24-0 | SDF | Download SDF |
| PubChem ID | 2930014 | Appearance | Powder |
| Formula | C13H15N3O2S | M.Wt | 277.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | K 858;K-858 | ||
| Solubility | DMSO : 100 mg/mL (360.57 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
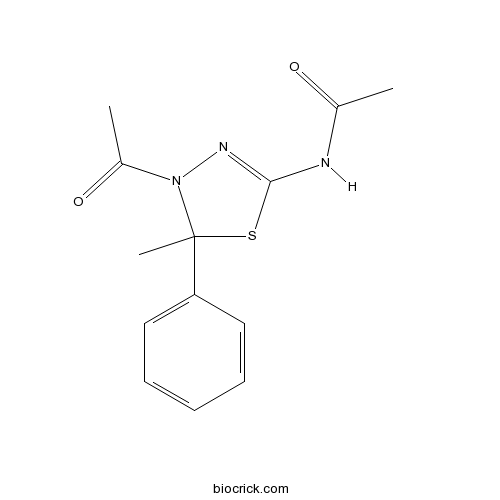
| Chemical Name | N-(4-acetyl-5-methyl-5-phenyl-1,3,4-thiadiazol-2-yl)acetamide | ||
| SMILES | CC(=O)NC1=NN(C(S1)(C)C2=CC=CC=C2)C(=O)C | ||
| Standard InChIKey | JEFVYQYZCAVNTP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H15N3O2S/c1-9(17)14-12-15-16(10(2)18)13(3,19-12)11-7-5-4-6-8-11/h4-8H,1-3H3,(H,14,15,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective ATP-uncompetitive mitotic kinesin Eg5 inhibitor (IC50 = 1.3 μM). Induces mitotic arrest, caspase-3 activation and cell growth inhibition in HCT116 cells. Displays no effect on microtubule dynamics. Preferentially induces mitotic arrest in cancer cells. |

K858 Dilution Calculator

K858 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6057 mL | 18.0284 mL | 36.0568 mL | 72.1137 mL | 90.1421 mL |
| 5 mM | 0.7211 mL | 3.6057 mL | 7.2114 mL | 14.4227 mL | 18.0284 mL |
| 10 mM | 0.3606 mL | 1.8028 mL | 3.6057 mL | 7.2114 mL | 9.0142 mL |
| 50 mM | 0.0721 mL | 0.3606 mL | 0.7211 mL | 1.4423 mL | 1.8028 mL |
| 100 mM | 0.0361 mL | 0.1803 mL | 0.3606 mL | 0.7211 mL | 0.9014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
K858 is an ATP-uncompetitive inhibitor of Eg5 with an IC50 of 1.3 μM.
In Vitro:K858 is an ATP-uncompetitive inhibitor of Eg5 with an IC50 of 1.3 μM. K858 does not inhibit the ATPase activity of the mitotic kinesins CENP-E and MKLP1, or the conventional kinesin heavy chain even at 200 μM. K858 induces mitotic arrest and growth inhibition via the activation of the Mad2-mediated spindle checkpoint. K858 (5 μM) induces mitotic cell death in cancer cells but not in normal cells[1]. K858 (1, 10, 100 μM) inhibits the MCF7, BT474 and SKBR3 cell lines, and only at 10 and 100 μM suppresses MDA-MB231 cell line after treatment for 24 h. K858 incereases Bax/Bcl2 RNA ratio and survivin in the four cell lines. Furthermore, the up-regulation of survivin is totally reversed by wortmannin (phosphoinositide 3-kinase AKT) in MCF7 cells[2].
In Vivo:K858 (50, 150 mg/kg, p.o.) shows antitumor activity in an A2780 ovarian cancer xenograft model, also inhibits tumor grwoth in a HCT116 colon cancer xenograft model via 100 mg/kg twice a day orally for 5 days. K858 (100 mg/kg, p.o., qd ×5) displays no neurotoxic side effects in mice[1].
References:
[1]. Nakai R, et al. K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res. 2009 May 1;69(9):3901-9.
[2]. De Iuliis F, et al. The kinesin Eg5 inhibitor K858 induces apoptosis but also survivin-related chemoresistance in breast cancer cells. Invest New Drugs. 2016 Aug;34(4):399-406.
- Bohemamine
Catalog No.:BCN1958
CAS No.:72926-12-6
- (RS)-4-Carboxyphenylglycine
Catalog No.:BCC6601
CAS No.:7292-81-1
- α,β-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC3591
CAS No.:7292-42-4
- (Z)-Butylidenephthalide
Catalog No.:BCN4007
CAS No.:72917-31-8
- O-Methylcedrelopsin
Catalog No.:BCN3637
CAS No.:72916-61-1
- Isocrocandine
Catalog No.:BCN2071
CAS No.:72903-70-9
- 4-Propenylbrenzcatechin
Catalog No.:BCN3672
CAS No.:72898-29-4
- Benzoyloxypeoniflorin
Catalog No.:BCN2799
CAS No.:72896-40-3
- CHIR-090
Catalog No.:BCC1477
CAS No.:728865-23-4
- Agatharesinol
Catalog No.:BCN4594
CAS No.:7288-11-1
- Crocandine
Catalog No.:BCN2070
CAS No.:72855-83-5
- Echitoveniline
Catalog No.:BCN7490
CAS No.:72855-79-9
- Octadecyl p-coumarate
Catalog No.:BCN7235
CAS No.:72943-88-5
- 30-Hydroxylup-20(29)-en-3-one
Catalog No.:BCN4286
CAS No.:72944-06-0
- DL-Catechin
Catalog No.:BCN6325
CAS No.:7295-85-4
- Carvedilol
Catalog No.:BCC4324
CAS No.:72956-09-3
- 2-Furoyl-LIGRLO-amide
Catalog No.:BCC3958
CAS No.:729589-58-6
- 6,7-Dihydroneridienone A
Catalog No.:BCN4020
CAS No.:72959-46-7
- Gomisin E
Catalog No.:BCN7031
CAS No.:72960-21-5
- Gomisin O
Catalog No.:BCN2875
CAS No.:72960-22-6
- XRP44X
Catalog No.:BCC7568
CAS No.:729605-21-4
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
Crystal structure of the Eg5 - K858 complex and implications for structure-based design of thiadiazole-containing inhibitors.[Pubmed:30031975]
Eur J Med Chem. 2018 Aug 5;156:641-651.
The thiadiazole scaffold is an important core moiety in a variety of clinical drug candidates targeting a range of diseases. For example, the 2,4,5-substituted 1,3,4-thiadiazole scaffold is present in a lead compound and at least two clinical candidates targeting the human motor protein Eg5, against neoplastic diseases. An inhibitor named K858 has in vivo activity in various mouse xenografts whereas the clinical candidates (S)-ARRY-520 and (R)-Litronesib have entered clinical trials with the former one in phase III clinical trials either alone or in combination with a proteasome inhibitor against relapsed/refractory multiple myeloma. Astonishingly, structural data are lacking for all thiadiazole-containing Eg5 inhibitors. Here we report the structure determination of two crystal forms of the ternary Eg5-ADP-K858 complex, locking the motor in the so-called final inhibitor bound state, thus blocking ADP release, a crucial stage for Eg5 activity. K858 acts at the established allosteric inhibitor-binding pocket formed of helix alpha2, loop L5 and helix alpha3. The structure of the complex has far reaching consequences for thiadiazole containing Eg5 inhibitors. For example, we could rationalise the structure-activity relationship in the crucial 5-position of the thiadiazole scaffold and the complex will serve in the future as a basis for strucutre-based drug design.
Growth arrest and apoptosis induced by kinesin Eg5 inhibitor K858 and by its 1,3,4-thiadiazoline analogue in tumor cells.[Pubmed:29738338]
Anticancer Drugs. 2018 Aug;29(7):674-681.
Tumors are complex and heterogeneous but, despite this, they share the ability to proliferate continuously, irrespective of the presence of growth signals, leading to a higher fraction of actively growing and dividing cells compared with normal tissues. For this reason, the cytotoxic antimitotic treatments remain an important clinical tool for tumors. Among these drugs, antitubulin compounds constitute one of the most effective anticancer chemotherapies; however, they cause dose-limiting side effects. Therefore, it is still necessary to develop compounds with new targets and new mechanisms of action to reduce side effects or chemoresistance. Mitosis-specific kinesin Eg5 can represent an attractive target for discovering such new anticancer agents because its role is fundamental in mitotic progression. Therefore, we analyzed the effects induced by an inhibitor of kinesin Eg5, K858, and by its 1,3,4-thiadiazoline analogue on human melanoma and prostate cancer cell lines. We found that both compounds have an antiproliferative effect, induce apoptosis, and can determine a downmodulation of survivin.
The kinesin Eg5 inhibitor K858 induces apoptosis and reverses the malignant invasive phenotype in human glioblastoma cells.[Pubmed:28965307]
Invest New Drugs. 2018 Feb;36(1):28-35.
Glioblastoma multiforme is the most common primary malignant brain tumor and its current chemotherapeutic options are limited to temozolomide. Recently, some synthetic compounds acting as inhibitors of kinesin spindle protein Eg5 have shown pronounced antitumor activity. Our group has recently demonstrated that one of these kinesin Eg5 inhibitors, named K858, exerted important antiproliferative and apoptotic effects on breast cancer cells. Since glioblastoma cells usually express high levels of kinesin Eg5, we tested the effect of K858 on two human glioblastoma cell lines (U-251 and U-87) and found that K858 inhibited cell growth, induced apoptosis, reversed epithelial-mesenchymal transition and inhibited migration in both cell lines. We also detected that, at the same time, K858 increased the expression of survivin, an anti-apoptotic molecule, and that the forced down-regulation of survivin, obtained with the specific inhibitor YM155, boosted K858-dependent apoptosis. This indicated that the anti-tumor activity of K858 on glioblastoma cells is limited by the over-expression of survivin and that the negative regulation of this protein sensitizes tumor cells to K858. These data confirmed that kinesin Eg5 is an interesting target for new therapeutic approaches for glioblastoma. We showed that K858, specifically, was a potent inhibitor of replication, an inducer of apoptosis and a negative regulator of the invasive phenotype for glioblastoma cells.
The kinesin Eg5 inhibitor K858 induces apoptosis but also survivin-related chemoresistance in breast cancer cells.[Pubmed:26994617]
Invest New Drugs. 2016 Aug;34(4):399-406.
Inhibitors of kinesin spindle protein Eg5 are characterized by pronounced antitumor activity. Our group has recently synthesized and screened a library of 1,3,4-thiadiazoline analogues with the pharmacophoric structure of K858, an Eg5 inhibitor. We herein report the effects of K858 on four different breast cancer cell lines: MCF7 (luminal A), BT474 (luminal B), SKBR3 (HER2 like) and MDA-MB231 (basal like). We demonstrated that K858 displayed anti-proliferative activity on every analyzed breast cancer cell line by inducing apoptosis. However, at the same time, we showed that K858 up-regulated survivin, an anti-apoptotic molecule. We then performed a negative regulation of survivin expression, with the utilization of wortmannin, an AKT inhibitor, and obtained a significant increase of K858-dependent apoptosis. These data demonstrate that K858 is a potent inhibitor of replication and induces apoptosis in breast tumor cells, independently from the tumor phenotype. This anti-proliferative response of tumor cells to K858 can be limited by the contemporaneous over-expression of survivin; consequently, the reduction of survivin levels, obtained with AKT inhibitors, can sensitize tumor cells to K858-induced apoptosis.
Synthesis and pharmacological screening of a large library of 1,3,4-thiadiazolines as innovative therapeutic tools for the treatment of prostate cancer and melanoma.[Pubmed:26498571]
Eur J Med Chem. 2015 Nov 13;105:245-62.
Antimitotic agents are widely used in cancer chemotherapy but the numerous side effects and the onset of resistance limit their clinical efficacy. Therefore, with the purpose of discovering more selective and efficient anticancer agents to be administered alone or in combination with traditional drugs, we synthesized a large library of 1,3,4-thiadiazoline analogues, maintaining the pharmacophoric structure of an antiproliferative compound known as K858: this is a new inhibitor of kinesin Eg5, able to induce the mitotic arrest in colorectal cancer cells and in xenograft ovarian cancer cells. We screened 103 compounds to assess their antiproliferative activity on PC3 prostate cancer cell line. Two derivatives, compounds 32 (corresponding to K858) and 33, have shown to be the most effective against prostate tumor cells and also towards two melanoma cell lines (SK-MEL-5 and SK-MEL-28) at low micromolar concentrations, confirming the pharmacological activity of this scaffold and revealing the potential role of 1,3,4-thiadiazolines in the management of cancer.
STLC-resistant cell lines as tools to classify chemically divergent Eg5 targeting agents according to their mode of action and target specificity.[Pubmed:24041742]
Biochem Pharmacol. 2013 Nov 15;86(10):1441-51.
Determining the mechanism of action of drugs and their target specificity in cells remains a major challenge. Here we describe the use of cell lines expressing two point mutations in the allosteric inhibitor binding pocket of the mitotic kinesin Eg5 (D130A, in the loop L5 region and L214A in helix alpha3), which following transfection, were selected for their ability to proliferate normally in the presence of STLC, a well known Eg5 inhibitor. The cell lines were used to discriminate the mechanism of action of other chemically distinct small molecule inhibitors of Eg5 that differ in their mode of action. The STLC resistant cells were capable of continuous proliferation in the presence of ATP uncompetitive inhibitors, such as K858 and dimethylenastron, but were still sensitive to ATP competitive inhibitors that are thought to bind to a distinct site on Eg5 than the allosteric binding pocket. The STLC resistant cell lines can therefore be used as a filter to distinguish Eg5 loop L5 binding drugs from drugs binding to other pockets without prior structural information. Additionally, the cells can be used to analyze whether inhibitors of Eg5 are specific to this potential drug target or whether they have additional targets in dividing cells.
K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells.[Pubmed:19351824]
Cancer Res. 2009 May 1;69(9):3901-9.
The aim of this study was to investigate the mechanism of inhibition of Eg5 (kinesin spindle protein), a mitotic kinesin that plays an essential role in establishing mitotic spindle bipolarity, by the novel small molecule inhibitor K858. K858 was selected in a phenotype-based forward chemical genetics screen as an antimitotic agent, and subsequently characterized as an inhibitor of Eg5. K858 blocked centrosome separation, activated the spindle checkpoint, and induced mitotic arrest in cells accompanied by the formation of monopolar spindles. Long-term continuous treatment of cancer cells with K858 resulted in antiproliferative effects through the induction of mitotic cell death, and polyploidization followed by senescence. In contrast, treatment of nontransformed cells with K858 resulted in mitotic slippage without cell death, and cell cycle arrest in G(1) phase in a tetraploid state. In contrast to paclitaxel, K858 did not induce the formation of micronuclei in either cancer or nontransformed cells, suggesting that K858 has minimal effects on abnormalities in the number and structure of chromosomes. K858 exhibited potent antitumor activity in xenograft models of cancer, and induced the accumulation of mitotic cells with monopolar spindles in tumor tissues. Importantly, K858, unlike antimicrotubule agents, had no effect on microtubule polymerization in cell-free and cell-based assays, and was not neurotoxic in a motor coordination test in mice. Taken together, the Eg5 inhibitor K858 represents an important compound for further investigation as a novel anticancer therapeutic.


