JNJ 5207852 dihydrochlorideHigh affinity H3 receptor antagonist CAS# 398473-34-2 |
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- SU11274
Catalog No.:BCC1243
CAS No.:658084-23-2
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 398473-34-2 | SDF | Download SDF |
| PubChem ID | 2766326 | Appearance | Powder |
| Formula | C20H32N2O | M.Wt | 316.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 77.5 mg/mL (244.88 mM; Need ultrasonic) | ||
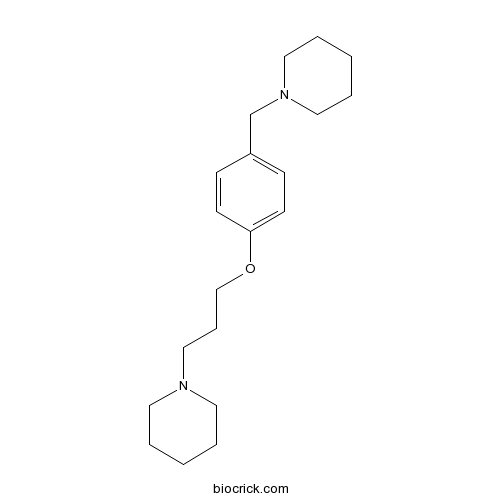
| Chemical Name | 1-[3-[4-(piperidin-1-ylmethyl)phenoxy]propyl]piperidine | ||
| SMILES | C1CCN(CC1)CCCOC2=CC=C(C=C2)CN3CCCCC3 | ||
| Standard InChIKey | PTKHFRNHJULJKT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity histamine H3 receptor neutral antagonist (pKi values are 8.9 and 9.2 in rat and human respectively). Brain penetrant and orally active. Has 3- and 100-fold higher affinity than thioperamide for rat and human H3 receptors respectively. Suppresses slow-wave sleep; exhibits wake-promoting effects in rodent arousal models. |

JNJ 5207852 dihydrochloride Dilution Calculator

JNJ 5207852 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1596 mL | 15.7978 mL | 31.5956 mL | 63.1912 mL | 78.9889 mL |
| 5 mM | 0.6319 mL | 3.1596 mL | 6.3191 mL | 12.6382 mL | 15.7978 mL |
| 10 mM | 0.316 mL | 1.5798 mL | 3.1596 mL | 6.3191 mL | 7.8989 mL |
| 50 mM | 0.0632 mL | 0.316 mL | 0.6319 mL | 1.2638 mL | 1.5798 mL |
| 100 mM | 0.0316 mL | 0.158 mL | 0.316 mL | 0.6319 mL | 0.7899 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 19-Hydroxybufalin
Catalog No.:BCN8237
CAS No.:39844-86-5
- Methyllinderone
Catalog No.:BCN5452
CAS No.:3984-73-4
- Amikacin disulfate
Catalog No.:BCC4622
CAS No.:39831-55-5
- H-Ala-OiPr.HCl
Catalog No.:BCC3193
CAS No.:39825-33-7
- Epitulipinolide diepoxide
Catalog No.:BCN5451
CAS No.:39815-40-2
- Taibaihenryiins A
Catalog No.:BCN3281
CAS No.:398129-59-4
- Penciclovir
Catalog No.:BCC4695
CAS No.:39809-25-1
- Azatadine dimaleate
Catalog No.:BCC4536
CAS No.:3978-86-7
- Boc-Tyr-OH
Catalog No.:BCC3458
CAS No.:3978-80-1
- 2-Amino-4-hydroxy-6-methylpyrimidine
Catalog No.:BCC8531
CAS No.:3977-29-5
- Bryonolol
Catalog No.:BCN2703
CAS No.:39765-50-9
- Dehydrovomifoliol
Catalog No.:BCN7562
CAS No.:39763-33-2
- PHA-680632
Catalog No.:BCC2178
CAS No.:398493-79-3
- H-D-Phe(4-OMe)-OH
Catalog No.:BCC2633
CAS No.:39878-65-4
- 29-Hydroxyfriedelan-3-one
Catalog No.:BCN5453
CAS No.:39903-21-4
- 2'-Deoxycytidine hydrochloride
Catalog No.:BCC5434
CAS No.:3992-42-5
- Norglaucine hydrochloride
Catalog No.:BCN6568
CAS No.:39945-41-0
- LY 78335
Catalog No.:BCC6109
CAS No.:39959-66-5
- Victoxinine
Catalog No.:BCN6745
CAS No.:39965-06-5
- HOAt
Catalog No.:BCC2815
CAS No.:39968-33-7
- Delta-9-Tetrahydrocannabivarinic acid
Catalog No.:BCN7967
CAS No.:39986-26-0
- p-Hydroxy-5,6-dehydrokawain
Catalog No.:BCN3597
CAS No.:39986-86-2
- Laricitrin 3-O-glucoside
Catalog No.:BCN8149
CAS No.:39986-90-8
- H-Thr-OMe.HCl
Catalog No.:BCC3104
CAS No.:39994-75-7
Histamine H3 Heteroreceptors Suppress Glutamatergic and GABAergic Synaptic Transmission in the Rat Insular Cortex.[Pubmed:29170631]
Front Neural Circuits. 2017 Nov 9;11:85.
Histamine H3 receptors are autoreceptors that regulate histamine release from histaminergic neuronal terminals. The cerebral cortex, including the insular cortex (IC), expresses abundant H3 receptors; however, the functions and mechanisms of H3 receptors remain unknown. The aim of this study was to elucidate the functional roles of H3 in synaptic transmission in layer V of the rat IC. Unitary excitatory and inhibitory postsynaptic currents (uEPSCs and uIPSCs) were obtained through paired whole-cell patch-clamp recording in cerebrocortical slice preparations. The H3 receptor agonist, R-alpha-methylhistamine (RAMH), reduced the uEPSC amplitude obtained from pyramidal cell to pyramidal cell or GABAergic interneuron connections. Similarly, RAMH reduced the uIPSC amplitude in GABAergic interneuron to pyramidal cell connections. RAMH-induced decreases in both the uEPSC and uIPSC amplitudes were accompanied by increases in the failure rate and paired-pulse ratio. JNJ 5207852 dihydrochloride or thioperamide, H3 receptor antagonists, inhibited RAMH-induced suppression of uEPSCs and uIPSCs. Unexpectedly, thioperamide alone increased the uIPSC amplitude, suggesting that thioperamide was likely to act as an inverse agonist. Miniature EPSC or IPSC recordings support the hypothesis that the activation of H3 receptors suppresses the release of glutamate and GABA from presynaptic terminals. The colocalization of H3 receptors and glutamate decarboxylase or vesicular glutamate transport protein 1 in presynaptic axon terminals was confirmed through double pre-embedding microscopy, using a combination of pre-embedding immunogold and immunoperoxidase techniques. The suppressive regulation of H3 heteroreceptors on synaptic transmission might mediate the regulation of sensory information processes, such as gustation and visceral sensation, in the IC.
Correlation between ex vivo receptor occupancy and wake-promoting activity of selective H3 receptor antagonists.[Pubmed:18305012]
J Pharmacol Exp Ther. 2008 Jun;325(3):902-9.
The histamine H3 receptor (H3R) modulates the release of neurotransmitters that are involved in vigilance, cognition, and sleep-wake regulation. H3R antagonism has been proposed as a novel approach to the treatment of cognitive and attention deficit as well as sleep disorders. It is apparent that H3R antagonists produce pharmacological effects in preclinical animal models across a wide dose range. Several H3R antagonists were reported to be effective at producing cognitive enhancing effects at low doses, while producing robust wake enhancement at higher doses. To better understand the effect of H3R antagonists across a broad dose range, an ex vivo receptor binding assay has been used to estimate the degree of H3R occupancy in vivo. The H3R antagonists ciproxifan, thioperamide, GSK189254 (6-[(3-cyclobutyl-2,3,4,5-tetrahydro-1H-3-benzazepin-7-yl)oxy]-N-methyl-3-pyridin ecarboxamide hydrochloride), and ABT-239 ([4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile) produced wake-promoting activity in vivo and a dose-dependent inhibition of H3R binding ex vivo. For ciproxifan, thioperamide, and GSK189254, a relatively low level of cumulative wake activity was linearly correlated with up to 80% of the receptor occupancy. In contrast, an abrupt break from linearity and a robust increase of waking activity was observed at doses that produce greater than 80% occupancy. Our results suggest a relatively small increase of waking activity at low levels of receptor occupancy that may be consistent with reported enhancement of attention and cognitive function. Robust waking activity at higher levels of H3R occupancy may be mechanistically different from activities at low levels of H3R occupancy.
Effects of histamine H(3) antagonists and donepezil on learning and mnemonic deficits induced by pentylenetetrazol kindling in weanling mice.[Pubmed:16310812]
Neuropharmacology. 2006 Mar;50(4):404-11.
Childhood epilepsy is one of the main risk factors for a variety of problems involving cognition and behavior. Pentylenetetrazol (PTZ) kindling is currently an acceptable model for epilepsy research. The objectives of this study are to clarify the learning and mnemonic characteristics of PTZ kindling in developing mice, and to examine the effects of thioperamide and JNJ-5207852, two histamine H(3) receptor antagonists and donepezil, an acetylcholinesterase (AChE) inhibitor, on learning and memory deficits induced by PTZ kindling in the brains of developing mice. PTZ kindling led to learning and mnemonic deficits as assessed by social discrimination, acoustic fear conditioning, water maze and passive avoidance tests. Thioperamide and JNJ-5207852, ameliorated PTZ kindling-induced learning and mnemonic deficits in all tests except for the water maze test. In addition, the learning and mnemonic impairments induced by PTZ kindling were significantly improved by donepezil in all tests. These findings suggest that histamine and acetylcholine are involved in the different processes of learning and memory in the brain and that histamine H(3) receptor antagonists might be useful in the treatment of cognitive impairment in epilepsy.
Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist.[Pubmed:15466448]
Br J Pharmacol. 2004 Nov;143(5):649-61.
1 1-[4-(3-piperidin-1-yl-propoxy)-benzyl]-piperidine (JNJ-5207852) is a novel, non-imidazole histamine H3 receptor antagonist, with high affinity at the rat (pKi=8.9) and human (pKi=9.24) H3 receptor. JNJ-5207852 is selective for the H3 receptor, with negligible binding to other receptors, transporters and ion channels at 1 microm. 2 JNJ-5207852 readily penetrates the brain tissue after subcutaneous (s.c.) administration, as determined by ex vivo autoradiography (ED50 of 0.13 mg kg(-1) in mice). In vitro autoradiography with 3H-JNJ-5207852 in mouse brain slices shows a binding pattern identical to that of 3H-R-alpha-methylhistamine, with high specific binding in the cortex, striatum and hypothalamus. No specific binding of 3H-JNJ-5207852 was observed in brains of H3 receptor knockout mice. 3 In mice and rats, JNJ-5207852 (1-10 mg kg(-1) s.c.) increases time spent awake and decreases REM sleep and slow-wave sleep, but fails to have an effect on wakefulness or sleep in H3 receptor knockout mice. No rebound hypersomnolence, as measured by slow-wave delta power, is observed. The wake-promoting effects of this H3 receptor antagonist are not associated with hypermotility. 4 A 4-week daily treatment of mice with JNJ-5207852 (10 mg kg(-1) i.p.) did not lead to a change in body weight, possibly due to the compound being a neutral antagonist at the H3 receptor. 5 JNJ-5207852 is extensively absorbed after oral administration and reaches high brain levels. 6 The data indicate that JNJ-5207852 is a novel, potent and selective H3 antagonist with good in vitro and in vivo efficacy, and confirm the wake-promoting effects of H3 receptor antagonists.


