Isovitexin-2''-O-rhamnosideCAS# 72036-50-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 72036-50-1 | SDF | File under preparation. |
| PubChem ID | 44257672 | Appearance | Powder |
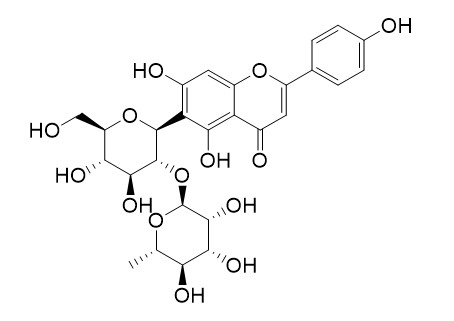
| Formula | C27H30O14 | M.Wt | 578.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 2''-O-alpha-L-Rhamnopyranosyl-isovitexin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-[(2S,4S,5S)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,4S,5R)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(C(OC2C3=C(C4=C(C=C3O)OC(=CC4=O)C5=CC=C(C=C5)O)O)CO)O)O)O)O)O | ||
| Standard InChIKey | BGPMMCPSTAYIEL-GKSGMORCSA-N | ||
| Standard InChI | InChI=1S/C27H30O14/c1-9-19(32)22(35)24(37)27(38-9)41-26-23(36)20(33)16(8-28)40-25(26)18-13(31)7-15-17(21(18)34)12(30)6-14(39-15)10-2-4-11(29)5-3-10/h2-7,9,16,19-20,22-29,31-37H,8H2,1H3/t9?,16?,19-,20+,22-,23-,24?,25-,26?,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isovitexin-2''-O-rhamnoside Dilution Calculator

Isovitexin-2''-O-rhamnoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7286 mL | 8.643 mL | 17.2861 mL | 34.5722 mL | 43.2152 mL |
| 5 mM | 0.3457 mL | 1.7286 mL | 3.4572 mL | 6.9144 mL | 8.643 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7286 mL | 3.4572 mL | 4.3215 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 9-Octadecenedioic acid
Catalog No.:BCN0193
CAS No.:4494-16-0
- 4,7-Didehydroneophysalin B
Catalog No.:BCN0192
CAS No.:134461-76-0
- Solafuranone
Catalog No.:BCN0191
CAS No.:367965-50-2
- Isomargaritene
Catalog No.:BCN0190
CAS No.:64271-11-0
- Rosmarinyl glucoside
Catalog No.:BCN0189
CAS No.:910028-78-3
- Fortunellin-6''-beta-D-glucopyranoside (Acacetin-7-O-[2''-O-rhamnosyl-6''-O-glucosyl]-glucoside)
Catalog No.:BCN0188
CAS No.:1218774-64-1
- Phloretin 3',5'-Di-C-glucoside
Catalog No.:BCN0187
CAS No.:357401-40-2
- Clemomandshuricoside B
Catalog No.:BCN0186
CAS No.:905294-48-6
- Glaucoside A
Catalog No.:BCN0185
CAS No.:81474-91-1
- 11-Deoxyisomogroside V
Catalog No.:BCN0184
CAS No.:1628293-32-2
- 3',5-Dihydroxy-4',6,7-trimethoxyflavanone
Catalog No.:BCN0183
CAS No.:90850-99-0
- Beta-Dimorphecolic acid (9(S)-HODE)
Catalog No.:BCN0182
CAS No.:18104-44-4
- 8'-O-(3-hydroxy-3-methylglutaryl)-8'-hydroxyabscisic acid
Catalog No.:BCN0195
CAS No.:69790-31-4
- Menisperine
Catalog No.:BCN0196
CAS No.:25342-82-9
- Batatasin V
Catalog No.:BCN0197
CAS No.:65817-45-0
- alpha-Costic acid
Catalog No.:BCN0198
CAS No.:28399-17-9
- Parishin G
Catalog No.:BCN0199
CAS No.:952283-93-1
- Arvenin III
Catalog No.:BCN0200
CAS No.:65597-45-7
- Physalin X
Catalog No.:BCN0201
CAS No.:72497-31-5
- Notoginsenoside L13
Catalog No.:BCN0202
CAS No.:2485859-56-9
- Euphorbia factor L24
Catalog No.:BCN0203
CAS No.:1613700-13-2
- Batatasin IV
Catalog No.:BCN0204
CAS No.:60347-67-3
- Cuscutamine
Catalog No.:BCN0205
CAS No.:122170-93-8
- Isocucurbitacin D
Catalog No.:BCN0206
CAS No.:68422-20-8
Isolation of C-glycosylflavonoids with alpha-glucosidase inhibitory activity from Passiflora bogotensis Benth by gradient high-speed counter-current chromatography.[Pubmed:25864011]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 May 15;990:104-10.
In this study, we applied a gradient High-Speed Counter-Current Chromatography (HSCCC) method that allowed, by direct injection of an aqueous crude extract of the leaves of Passiflora bogotensis, the successful isolation of six flavonoids in a single run, with purity of each compound higher than 81%. This separation enabled the isolation of two new flavonoid glycosides, apigenin-6-C-alpha-l-rhamnopyranosyl-(1-->2)-(6''-O-acetyl)-beta-d-glucopyranosid e (2) and luteolin-6-C-alpha-l-rhamnopyranosyl-(1-->2)-(6''-O-acetyl)-beta-d-glucopyranosid e (4), and four known ones, isovitexin (1), isoorientin (3), isovitexin-2''-O-rhamnoside (5) and isoorientin-2''-O-rhamnoside (6). The structures of the isolated compounds were identified by HPLC-DAD, LC-MS, (1)H and (13)C NMR and comparison with literature data. The inhibitory activities of all of these compounds were evaluated in vitro on alpha-glucosidase from S. cerevisiae, and the IC50 was determinate. This is the first study concerning the chemical composition and biological activity of Passiflora bogotensis.
Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities.[Pubmed:24151872]
J Agric Food Chem. 2013 Nov 6;61(44):10507-15.
The phenolic profiles of Tetrastigma hemsleyanum leaf extracts by different solvents (80% methanol, ethyl acetate and hexane) and their antioxidant and antiproliferative activities were investigated. Thirteen phenolic compounds (3-caffeoylquinic acid, 5-caffeoylquinic acid, 1-caffeoylquinic acid, 5-p-coumaroylquinic acid, isoorientin-2''-O-rhamnoside, isoorientin, orientin-2''-O-rhamnoside, orientin, 1-p-coumaroylquinic acid, vitexin-2''-O-rhamnoside, isovitexin-2''-O-rhamnoside, vitexin and isovitexin) were identified in T. hemsleyanum leaves for the first time, and six of them were quantified using a combination of LC-QTOF-MS and LC-QqQ-MS techniques. It was found that 80% methanol extract exhibited the highest antioxidant activities (DPPH, 3.32 mmol of Trolox/g DW; ABTS, 1.38 mmol of Trolox/g DW; FRAP, 1.85 mmol of FeSO4/g DW), while the hexane extract had the lowest (1.23, 0.43 and 0.13, respectively). Total phenolic contents (TPC) of various extracts of T. hemsleyanum leaves ranged from 28.95 to 275.71 mg of GAE/g DW. Also, total antioxidant activities as evaluated by ABTS, FRAP and DPPH assays were correlated well with TPC. In addition, 80% methanol extract provided antiproliferative activity on HepG2 cells (IC50 = 524 mug/mL). This paper provides a complete picture of phenolics in T. hemsleyanum leaves and relates them to their antioxidant and antiproliferative activities.
Flavonoid C- and O-glycosides from the Mongolian medicinal plant Dianthus versicolor Fisch.[Pubmed:21640336]
Carbohydr Res. 2011 Sep 27;346(13):1868-75.
Eighteen flavonoids were identified from an aqueous extract of the aerial parts of Dianthus versicolor, a plant used in traditional Mongolian medicine against liver diseases. The flavonoid C- and O-glycosides isoorientin-7-O-rutinoside, isoorientin-7-O-rhamnosyl-galactoside, isovitexin-7-O-rutinoside, isovitexin-7-O-rhamnosyl-galactoside, isoscoparin-7-O-rutinoside, isoscoparin-7-O-rhamnosyl-galactoside, isoscoparin-7-O-galactoside, and isoorientin-7-O-galactoside were isolated and structurally elucidated. Their structures were established on the basis of extensive spectroscopic techniques including LC-UV-DAD, LC-MS(n), LC-HRMS, 1D and 2D NMR spectroscopy, and by GC-MS analysis after hydrolysis. Flavonoids with such a high glycosylation pattern are rare within the genus Dianthus. Furthermore, isovitexin-7-O-glucoside (saponarin), isovitexin-2''-O-rhamnoside, apigenin-6-glucoside (isovitexin), luteolin-7-O-glucoside, apigenin-7-O-glucoside, as well as the aglycons luteolin, apigenin, chrysoeriol, diosmetin, and acacetin were identified by TLC and LC-DAD-MS(n) in comparison to reference substances or literature data. The NMR data of seven structures have not been reported in the literature to date.
4'''-Acetylvitexin-2''-O-rhamnoside, isoorientin, orientin, and 8-methoxykaempferol-3-O-glucoside as markers for the differentiation of Crataegus monogyna and Crataegus pentagyna from Crataegus laevigata (Rosaceae).[Pubmed:18081102]
Chem Biodivers. 2007 Dec;4(12):2920-31.
In our chemotaxonomic investigation of pharmaceutically relevant Crataegus species, the qualitative and quantitative flavonoid fingerprint of Crataegus monogyna and C. pentagyna is presented. Six flavonoids were identified as vitexin-2''-O-rhamnoside (1), vitexin (2), isovitexin (3), rutin (4), hyperoside (5), and isoquercitrin (6). Besides the verification of the main compounds isoorientin (7) and orientin (8) in C. pentagyna, further four flavonoids were isolated and identified as isoorientin-2''-O-rhamnoside (9), orientin-2''-O-rhamnoside (10), isovitexin-2''-O-rhamnoside (11), and 8-methoxykaempferol-3-O-glucoside (12) by means of 1D- and 2D-NMR, MS, and UV analyses. Compound 12 was isolated for the first time from C. pentagyna. In contrast to C. pentagyna, C. monogyna samples were predominated by 4'''-acetylvitexin-2''-O-rhamnoside (13), which was missing in C. pentagyna. Hence, 13 represents an interesting compound for chemotaxonomy of C. monogyna, whereas the main flavonoids 7, 8, and 12 could be proposed as markers for C. pentagyna. The absence of 7, 8, 12, and 13 in C. laevigata offers an appropriate tool for additional differentiation from C. monogyna and C. pentagyna, and for sample identification and quality control of the three main Crataegus species used in European phytotherapy.


