IsooxypeucedaninCAS# 5058-15-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5058-15-1 | SDF | Download SDF |
| PubChem ID | 625383.0 | Appearance | Powder |
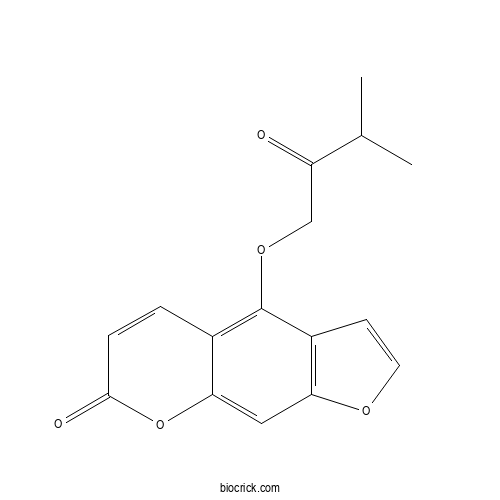
| Formula | C16H14O5 | M.Wt | 286.28 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-(3-methyl-2-oxobutoxy)furo[3,2-g]chromen-7-one | ||
| SMILES | CC(C)C(=O)COC1=C2C=CC(=O)OC2=CC3=C1C=CO3 | ||
| Standard InChIKey | USLPJJIUMAKBIU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O5/c1-9(2)12(17)8-20-16-10-3-4-15(18)21-14(10)7-13-11(16)5-6-19-13/h3-7,9H,8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isooxypeucedanin Dilution Calculator

Isooxypeucedanin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4931 mL | 17.4654 mL | 34.9308 mL | 69.8617 mL | 87.3271 mL |
| 5 mM | 0.6986 mL | 3.4931 mL | 6.9862 mL | 13.9723 mL | 17.4654 mL |
| 10 mM | 0.3493 mL | 1.7465 mL | 3.4931 mL | 6.9862 mL | 8.7327 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6986 mL | 1.3972 mL | 1.7465 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3493 mL | 0.6986 mL | 0.8733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxypeucedanin hydrate-3”-ethyl ether
Catalog No.:BCX1311
CAS No.:55481-87-3
- Theasaponin E2
Catalog No.:BCX1310
CAS No.:220114-30-7
- 3'-O-Angeloylhamaudol
Catalog No.:BCX1309
CAS No.:84272-84-4
- Entadamide A
Catalog No.:BCX1308
CAS No.:100477-88-1
- 13α,21-Dihydroeurycomanone
Catalog No.:BCX1307
CAS No.:129587-06-0
- Shinjulactone M
Catalog No.:BCX1306
CAS No.:103630-27-9
- Corymbiferin
Catalog No.:BCX1305
CAS No.:5042-09-1
- Poststerone
Catalog No.:BCX1304
CAS No.:10162-99-9
- Lyciumin B
Catalog No.:BCX1303
CAS No.:125756-66-3
- Theasaponin E1
Catalog No.:BCX1302
CAS No.:220114-28-3
- Kakkanin
Catalog No.:BCX1301
CAS No.:63770-91-2
- 16α-hydroxy-3-oxo-lanosta-7,9(11),24-trien-21-oic acid
Catalog No.:BCX1300
CAS No.:862109-64-6
- Poricoic acid BM
Catalog No.:BCX1313
CAS No.:1815623-74-5
- Neoartanin
Catalog No.:BCX1314
CAS No.:104196-69-2
- Rabdosia acid A
Catalog No.:BCX1315
CAS No.:1884697-13-5
- Tunicoidine A
Catalog No.:BCX1316
CAS No.:1415979-39-3
- Ethyl ganoderenate D
Catalog No.:BCX1317
CAS No.:2226858-23-5
- Ethyl ganoderate A
Catalog No.:BCX1318
CAS No.:2242593-18-4
- Anthranoyllycoctonine
Catalog No.:BCX1319
CAS No.:22413-78-1
- 3',4',7-Trimethoxyquercetin
Catalog No.:BCX1320
CAS No.:6068-80-0
- Wushanicaritin
Catalog No.:BCX1321
CAS No.:521-45-9
- 5-MethoxyPinocembroside
Catalog No.:BCX1322
CAS No.:1450878-89-3
- Decuroside V
Catalog No.:BCX1323
CAS No.:96648-59-8
- Anhydronotoptol
Catalog No.:BCX1324
CAS No.:88206-51-3
Tissue distribution study of Angelica dahurica cv. Yubaizhi in rat by ultra-performance liquid chromatography with tandem mass spectrometry.[Pubmed:31153136]
J Pharm Biomed Anal. 2019 Sep 10;174:43-49.
A sensitive and specific ultra-performance liquid chromatographic-tandem mass (UPLC-MS/MS) spectrometric method was established to investigate tissue distribution of fourteen coumarins of Angelica Dahurica cv. Yubaizhi roots (ADYR) in rat tissues, including isoimperatorin (1), imperatorin (2), Isooxypeucedanin (3), byakangelicin (4), oxypeucedanin hydrate (5), bergapten (6), 2"R-neobyakangelicol (7), phellopterin (8), xanthotoxin (9), isopimpinellin (10), oxypeucedanin ethanolate (11), isobyakangelicol (12), columbianetin (13), (-)-marmesin (14). Detection was performed on a triple quadrupole mass spectrometer in multiple-reaction-mode (MRM). The method established in this assay was successfully applied to tissue distribution study of the selected 14 coumarins after oral administration of the extract of ADYR in rat tissues, including heart, liver, spleen, lung, kidney, stomach, small intestine, muscle, testis, and brain. Tissue distribution characteristics of the fourteen coumarins were clearly elucidated, and the results of this study indicated that the fourteen coumarins were distributed to rat tissues rapidly and could be detected in all of the selected tissues after oral administration. Concentrations of the coumarins were obviously higher in kidney, liver and stomach tissues, and lower in testis, brain and muscle tissues. As an important part of ADMET/Act. study on ADYR, the tissue distribution of multiple coumarins of ADYR in rats provides a significant basis for better evaluation of the metabolism and disposition process in vivo of the herb medicine. The information provided in this research is very useful for further understanding of the metabolic mechanism of ADYR in vivo.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica cv.Yubaizhi].[Pubmed:28822155]
Zhongguo Zhong Yao Za Zhi. 2017 Jun;42(11):2102-2109.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica cv. Yubaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Thirty-three compounds were obtained and identified as isoimperatorin (1), imperatorin (2), stigmasterol (3), Isooxypeucedanin (4), pabulenol (5), psoralen (6), bergapten (7), isodemethylfuropinarine (8), phellopterin (9), osthenol (10), alloimperatorin (11), xanthotoxin (12), xanthotoxol (13), isopimpinellin (14), alloisoimperatorin (15), beta-sitosterol (16), oxyalloimperatorin (17), pabularinone (18), 5-hydroxy-8-methoxypsoralen (19), columbianetin (20), heracol (21), isogosferol (22), 2''R-neobyakangelicol (23), byakangelicin ethoxide (24), byakangelicin (25), oxypeucedanin hydrate (26), uracil (27), umbelliferone (28), bergaptol (29), demethylfuropinarine (30), isobyakangelicol (31), oxypeucedanin ethanolate (32), heraclenol (33). Among them, compounds 8, 10, 17, 21, and 30 were obtained from the roots of title plant for the first time.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi].[Pubmed:26552172]
Zhongguo Zhong Yao Za Zhi. 2015 Jun;40(11):2148-56.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica var. formosana cv. Chuanbaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Twenty-nine compounds were obtained and identified as isoimperatorin (1), beta-sitosterol (2), imperatorin (3), bergapten (4), osthenol (5), xanthotoxin (6), isoimpinellin (7), dehydrogeijerin (8), phellopterin (9), isodemethylfuropinarine (10), 7-demethylsuberosin (11), alloimperatorin (12), xanthotoxol (13), Isooxypeucedanin (14), alloisoimperatorin (15), demethylfuropinarine (16), 5-hydroxy-8-methoxypsoralen (17), oxypeucedanin methanolate (18), pabulenol (19), byakangelicin (20), marmesin (21), (+) -decursinol (22), heraclenol (23), oxypeucedanin hydrate (24), marmesinin (25), ulopterol (26), erythro-guaiacylglycerol-beta-ferulic acid ether (27), threo-guaiacylglycerol-beta-ferulic acid ether (28), and uracil (29). Compounds 5, 8, 11, 18, 21-23, and 26-28 were obtained from the roots of title plant for the first time.
Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins.[Pubmed:24800231]
Biomed Res Int. 2014;2014:480545.
Aim. Ducrosia anethifolia is used as flavoring additive. There have been little detailed phytochemical reports on this genus and the antidiabetic activity of this plant is not yet evaluated. Method. Structure of compounds was deduced by spectroscopic analyses. Preliminary in vitro evaluation of the antidiabetic activity of crude extract and its furanocoumarins was carried out ( alpha -amylase, alpha -glucosidase, and beta -galactosidase). The in vivo activity was investigated by measuring some oxidative stress markers. Biomarkers of liver injury and kidney were also determined. Results. Eight linear furanocoumarins, psoralen, 5-methoxypsoralen, 8-methoxypsoralen, imperatorin, Isooxypeucedanin, pabulenol, oxypeucedanin methanolate, oxypeucedanin hydrate, and 3-O-glucopyranosyl- beta -sitosterol, were isolated. All compounds were reported for the first time from the genus Ducrosia except pabulenol. The blood glucose level, liver function enzymes, total protein, lipid, and cholesterol levels were significantly normalized by extract treatment. The antioxidant markers, glucolytic, and gluconeogenic enzymes were significantly ameliorated and the elevated level of kidney biomarkers in the diabetic groups was restored. The compounds showed inhibitory activity in a concentration dependant manner. Imperatorin and 5-methoxypsoralen showed the most potent inhibiting power. Conclusion. D. anethifolia extract showed hypoglycemic, hypolipidemic, and antioxidant effect as well as ameliorating kidney function. This extract and some linear furanocoumarins exhibited carbohydrate metabolizing enzymes inhibitory effect.
[Chemical constituents from the roots of Angelica polymorpha Maxim].[Pubmed:23888695]
Yao Xue Xue Bao. 2013 May;48(5):718-22.
Angelica polymorpha Maxim. is a plant of the Angelica genus (Umbelliferae). The root and stem of this plant is a folk medicine known to have the actions of relieving rheumatism and cold and subsiding swelling and pains. To investigate the chemical constituents in the root of A. polymorpha Maxim., seven compounds were isolated from an 80% ethanol extract by column chromatography. Their structures were elucidated according to the spectroscopic analysis. Compound 1 is a new sesquiterpene, named as bisabolactone. Its absolute configuration was determined by 1D NOESY and CD analysis. The others were identified as 5-hydroxymethylfurfural (2), hycandinic acid ester 1 (3), ferulic acid (4), Isooxypeucedanin (5), noreugenin (6) and cimifugin (7). Compound 2 and 3 were isolated from this genus for the first time and compound 4 was isolated from this plant for the first time.
Supercritical fluid extraction for identification and determination of volatile metabolites from Angelica dahurica by GC-MS.[Pubmed:18705001]
J Sep Sci. 2008 Oct;31(18):3218-24.
The volatile components of Angelica dahurica were obtained by supercritical fluid extraction (SFE) method. These oils obtained were analyzed by GC-MS (identification and determination of metabolites). The compounds were identified according to their retention indices and mass spectra (electron impact (EI), 70 eV). The effects of different parameters, such as pressure, temperature, flow rate of CO(2), and the amount of modifier, on the SFE of A. dahurica oil were investigated. A total of 50 compounds of SFE extracts were identified. Phellopterin (PO), isoimperatorin (IO), imperatorin (IM), alloimperatorin (AM), byakangelicin, Isooxypeucedanin, and pimpinellin were the major coumarin compounds identified in A. dahurica SFE extracts. The quantitations of PO, IO, IM, and AM were then accomplished. The calibration curves showed good linearity (R(2) >0.99) in the concentration ranges tested. The recoveries were higher than 85%, with RSDs less than 10%. The GC-MS method was successfully validated and applied to the quantitation of A. dahurica.
Nonvolatiles of commercial lime and grapefruit oils separated by high-speed countercurrent chromatography.[Pubmed:16536603]
J Agric Food Chem. 2006 Mar 22;54(6):2242-52.
The nonvolatile fractions of cold-pressed peel oils of Key and Persian lime as well as grapefruit were separated by high-speed countercurrent chromatography (HS-CCC). In addition to the isolation of the main coumarins, psoralens and polymethoxyflavones, a number of minor constituents were enriched and successfully characterized by GC-MS and HPLC-UV. 5,7,8-Trimethoxycoumarin and the cyclical acetals of oxypeucedanin hydrate with citral were determined as new nonvolatile trace constituents of lime oils and confirmed by NMR spectroscopy. The citral oxypeucedaninyl acetals were found particularly in Key lime oil type A, which as a result of the juice-oil contact, is exposed to acidic conditions during industrial processing. Some of the confirmed minor constituents, such as pabulenol, Isooxypeucedanin, and oxypeucedanin methanolate in lime as well as auraptenol in grapefruit, may have been generated by hydrolysis-sensitive precursors during CCC separation or their respective industrial processing techniques.
Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A. gigas.[Pubmed:14560920]
Arch Pharm Res. 2003 Sep;26(9):723-6.
Five coumarins, isoimperatorin (1), pabulenol (2), Isooxypeucedanin (3), oxypeucedanin hydrate (4) and osthol (5) were isolated from the MeOH extract of Angelica genuflexa in the course of searching for anti-platelet and anti-coagulant components from plants. Pabulenol (2) was isolated from A. genuflexa for the first time. The five compounds isolated from A. genuflexa, together with decursinol angelate (6), decursin (7) and nodakenin (8) from A. gigas were evaluated for their effects on platelet aggregation and blood coagulation. Compounds 2, 5, 6 and 7 were observed to be either equally effective or 2-4 times more inhibitory than ASA in both arachidonic acid and U46619 (TXA2 mimetic) induced platelet aggregations.
Inhibitors of 5alpha -reductase type I in LNCaP cells from the roots of Angelica koreana.[Pubmed:11859469]
Planta Med. 2002 Feb;68(2):162-3.
A prenylated coumarin, osthenol (1) and a sesquiterpene, bisabolangelone (2) have been isolated as active principles with 5alpha-reductase type I inhibitory effects in LNCaP cells from the roots of Angelica koreana Max. by bioassay-guided chromatographic fractionation. Osthenol exhibited a highly potent inhibitory activity on 5alpha-reductase type I in LNCaP cells with an IC50 value of 0.1 microg/ml, which is about 200 times more potent than the positive control, finasteride (IC50 = 19.8 microg/ml). Bisabolangelone also inhibited the activity of 5alpha-reductase type I in LNCaP cells (IC50 = 11.6 microg/ml), indicating that these compounds are possible candidates for the development of new drugs to treat human endocrine disorders associated with overproduction of DHT by 5 alpha-reductase type I. In addition, four compounds Isooxypeucedanin, oxypeucedanin hydrate, oxypeucedanin and isoimperatorin were also isolated and found to be inactive in the 5alpha-reductase assay systems used in the present study.


