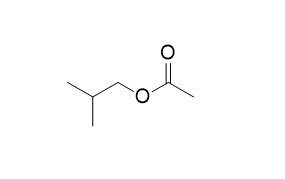
Isobutyl acetateCAS# 110-19-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 110-19-0 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Oil |
| Formula | C6H12O2 | M.Wt | 116.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Isobutyl acetate Dilution Calculator

Isobutyl acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6133 mL | 43.0663 mL | 86.1326 mL | 172.2653 mL | 215.3316 mL |
| 5 mM | 1.7227 mL | 8.6133 mL | 17.2265 mL | 34.4531 mL | 43.0663 mL |
| 10 mM | 0.8613 mL | 4.3066 mL | 8.6133 mL | 17.2265 mL | 21.5332 mL |
| 50 mM | 0.1723 mL | 0.8613 mL | 1.7227 mL | 3.4453 mL | 4.3066 mL |
| 100 mM | 0.0861 mL | 0.4307 mL | 0.8613 mL | 1.7227 mL | 2.1533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Urushiol (15:2)
Catalog No.:BCN9836
CAS No.:83258-37-1
- 6,7-Bis(benzyloxy)coumarin
Catalog No.:BCN9835
CAS No.:909-84-2
- BIX 01294 Trihydrochloride
Catalog No.:BCN9834
CAS No.:1392399-03-9
- Teupolioside
Catalog No.:BCN9833
CAS No.:143617-02-1
- Ethyl caproate
Catalog No.:BCN9832
CAS No.:123-66-0
- 28-Homobrassinolide
Catalog No.:BCN9831
CAS No.:82373-95-3
- Urushiol (15:1)
Catalog No.:BCN9830
CAS No.:35237-02-6
- 4,4'-Dimethoxychalcone
Catalog No.:BCN9829
CAS No.:2373-89-9
- Picrotoxinin
Catalog No.:BCN9828
CAS No.:17617-45-7
- 6-Hydroxyflavone
Catalog No.:BCN9827
CAS No.:6665-83-4
- Vaccarin E
Catalog No.:BCN9826
CAS No.:2252345-81-4
- Grayanotoxin I
Catalog No.:BCN9825
CAS No.:4720-09-6
- 5-Methoxyflavone
Catalog No.:BCN9838
CAS No.:42079-78-7
- Pilocarpine
Catalog No.:BCN9839
CAS No.:92-13-7
- Sutherlandioside D
Catalog No.:BCN9840
CAS No.:1055329-49-1
- Bletilol B
Catalog No.:BCN9841
CAS No.:147235-17-4
- Butyl acetate
Catalog No.:BCN9842
CAS No.:123-86-4
- Eugenol benzoate
Catalog No.:BCN9843
CAS No.:531-26-0
- Tryptanthrine
Catalog No.:BCN9844
CAS No.:13220-57-0
- 9-Hydroxy-O-senecioyl-8,9-dihydrooroselol
Catalog No.:BCN9845
CAS No.:31456-63-0
- Vitexin 7-glucoside
Catalog No.:BCN9846
CAS No.:35109-95-6
- Urushiol (15:3)
Catalog No.:BCN9847
CAS No.:83543-37-7
- Gardenin A
Catalog No.:BCN9848
CAS No.:21187-73-5
- alpha-Ionone
Catalog No.:BCN9849
CAS No.:127-41-3
Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits.[Pubmed:32950150]
Food Microbiol. 2020 Dec;92:103556.
Rotting caused by grey mould (Botrytis cinerea) is a concerning disease for numerous crops both pre- and postharvest stages. Application of antagonistic yeasts is a promising strategy for controlling grey mould incidence which could mitigate undesirable consequences of using synthetic fungicides. In this work, a screening for detection of yeasts isolated from figs producers of antifungal volatile organic compounds (VOCs) were performed by confrontation in double dishes systems. Eleven out of 34 yeasts confronted reduced B. cinerea growth parameter in vitro. This reduction was correlated (p Isobutyl acetate, 2-methylbutyl acetate, furfuryl acetate, phenylmethyl acetate, 2-phenylethyl acetate) and one ketone (Heptan-2-one). In bases on in vitro assay, Hanseniaspora uvarum 793 was applied to in vivo assays with strawberries and cherries. The reduction of incidence of B. cinerea in strawberries at 7 degrees C and 25 degrees C was 54.9 and 72.1% after 6 and 3 days, respectively. The reduction of incidence of B. cinerea in cherries at 7 degrees C and 25 degrees C was 48.9 and 45.6% after 5 and 4 days, respectively. These results showed that VOCs produced by Hanseniaspora uvarum 793 are effective in the control of incidence of Botrytis cinerea in fruits, being a potential alternative to chemical fungicide.
Clostridium thermocellum: A microbial platform for high-value chemical production from lignocellulose.[Pubmed:32948265]
Adv Appl Microbiol. 2020;113:111-161.
Second generation biorefining, namely fermentation processes based on lignocellulosic feedstocks, has attracted tremendous interest (owing to the large availability and low cost of this biomass) as a strategy to produce biofuels and commodity chemicals that is an alternative to oil refining. However, the innate recalcitrance of lignocellulose has slowed progress toward economically viable processes. Consolidated bioprocessing (CBP), i.e., single-step fermentation of lignocellulose may dramatically reduce the current costs of 2nd generation biorefining. Metabolic engineering has been used as a tool to develop improved microbial strains supporting CBP. Clostridium thermocellum is among the most efficient cellulose degraders isolated so far and one of the most promising host organisms for application of CBP. The development of efficient and reliable genetic tools has allowed significant progress in metabolic engineering of this strain aimed at expanding the panel of growth substrates and improving the production of a number of commodity chemicals of industrial interest such as ethanol, butanol, isobutanol, Isobutyl acetate and lactic acid. The present review aims to summarize recent developments in metabolic engineering of this organism which currently represents a reference model for the development of biocatalysts for 2nd generation biorefining.
The Role of the Bacterial Community in Producing a Peculiar Smell in Chinese Fermented Sour Soup.[Pubmed:32825573]
Microorganisms. 2020 Aug 21;8(9). pii: microorganisms8091270.
In this paper, the volatile flavour constituents and the bacterial diversity in characteristic Chinese fermented sour soup were analysed, and the dynamics of bacteria associated with the odour were characterized. The bacterial diversity of sour soup was studied by high-throughput sequencing. A total of 10 phyla and 89 genera were detected. Firmicutes was the dominant phylum of sour soup, accounting for 87.14-98.57%. The genus structure of normal sour soup was relatively simple, and Lactobacillus (78.05-90.26%) was the dominant genus. In addition to Lactobacillus, the foul-smelling sour soup contained more Pediococcus spp., Caproiciproducens spp., and Clostridium-sensu-stricto12 spp. (relative abundance >1%) than the normal sour soup. A total of 51 aroma compounds were detected by gas chromatography-mass spectrometry(GC-IMS), including 25 esters, 8 terpenes, 8 alcohols, 3 sulfur compounds, 2 acids, 2 ketones, 1 pyrazine, 1 monoterpene and 1 aldehyde. According to the relative odour active value (ROAV) calculation, 51 important flavour-contributing substances and 7 flavour-coordinating substances were determined. The esters with the highest relative percentages and ROAV values provided the pleasant flavour of the sour soup. In the foul-smelling sour soup, the ROAV values of 1,8-cineole, Isobutyl acetate, ethyl butanoate, ethyl octanoate-M, and ethyl hexanoate-M decreased, while those of diallyl disulfide-M and diallyl disulfide-D, which were probably responsible for the foul flavour, increased. Through Pearson correlation analysis, the odour production of the foul-smelling soup was determined to be related to Pediococcus spp., Caproiciproducens spp., Clostridiumsensu_stricto_12 spp., Oscillibacter spp., Bacteroides spp., Fibaculaceae_unclassified spp., Acinetobacter spp. and Halomonas spp.
Endogenous carbohydrate esterases of Clostridium thermocellum are identified and disrupted for enhanced isobutyl acetate production from cellulose.[Pubmed:32333614]
Biotechnol Bioeng. 2020 Jul;117(7):2223-2236.
Medium-chain esters are versatile chemicals with broad applications as flavors, fragrances, solvents, and potential drop-in biofuels. Currently, these esters are largely produced by the conventional chemical process that uses harsh operating conditions and requires high energy input. Alternatively, the microbial conversion route has recently emerged as a promising platform for sustainable and renewable ester production. The ester biosynthesis pathways can utilize either lipases or alcohol acyltransferase (AAT), but the AAT-dependent pathway is more thermodynamically favorable in an aqueous fermentation environment. Even though a cellulolytic thermophile Clostridium thermocellum harboring an AAT-dependent pathway has recently been engineered for direct conversion of lignocellulosic biomass into esters, the production is not efficient. One potential bottleneck is the ester degradation caused by the endogenous carbohydrate esterases (CEs) whose functional roles are poorly understood. The challenge is to identify and disrupt CEs that can alleviate ester degradation while not negatively affecting the efficient and robust capability of C. thermocellum for lignocellulosic biomass deconstruction. In this study, by using bioinformatics, comparative genomics, and enzymatic analysis to screen a library of CEs, we identified and disrupted the two most critical CEs, Clo1313_0613 and Clo1313_0693, that significantly contribute to Isobutyl acetate degradation in C. thermocellum. We demonstrated that an engineered esterase-deficient C. thermocellum strain not only reduced ester hydrolysis but also improved Isobutyl acetate production while maintaining effective cellulose assimilation.
Green Synthesis of the Flavor Esters with a Marine Candida parapsilosis Esterase Expressed in Saccharomyces cerevisiae.[Pubmed:32255850]
Indian J Microbiol. 2020 Jun;60(2):175-181.
The green synthesis of the flavor esters, n-propyl acetate, Isobutyl acetate and isoamyl acetate had the advantages over the chemical synthesis. The esterase from Candida parapsilosis could transform n-propanol, isobutanol and isoamyl alcohol into n-propyl acetate, Isobutyl acetate and isoamyl acetate, respectively. The esterase was expressed in Saccharomyces cerevisiae. At 30 degrees C for 1 d, the concentration of n-propyl acetate, Isobutyl acetate and isoamyl acetate synthesized by the esterase expressed in Saccharomyces cerevisiae was 24.6 mg/100 mL, 8.3 mg/100 mL, 5.6 mg/100 mL, respectively. Expression of the esterase has a practical significance for flavor ester synthesis by green biochemical process.
Olive Fruit Fly, Bactrocera oleae (Diptera: Tephritidae), Attraction to Volatile Compounds Produced by Host and Insect-Associated Yeast Strains.[Pubmed:31879768]
J Econ Entomol. 2020 Apr 6;113(2):752-759.
The olive fruit fly, Bactrocera oleae (Rossi), is one of the most damaging insect pests of olives worldwide, requiring the use of insecticides for fruit protection in many orchards. Olive fruit flies are attracted to volatile composunds, including a female-produced pheromone, and host-plant and bacterial volatiles. Preliminary laboratory bioassays were conducted for olive fruit fly attraction to over 130 yeast strains from among 400 that were isolated from B. oleae adults and larvae or other insects, infested olives, and potential feeding sites. Kuraishia capsulata, Scheffersomyces ergatensis, Peterozyma xylosa, Wickerhamomyces subpelliculosus, and Lachancea thermotolerans appeared to attract B. oleae as well or better than did torula yeast pellets (Cyberlindnera jadinii; syn. Candida utilis). Volatile compounds emitted by these yeast strains were chemically identified, and included isobutanol, isoamyl alcohol, 2-phenethyl alcohol, Isobutyl acetate, and 2-phenethyl acetate. The behavioral response of B. oleae adults to these volatile compounds at three concentrations was tested in a laboratory Y-tube olfactometer. The same volatile compounds were also tested in the field. Isoamyl alcohol was more attractive than the other compounds tested in both laboratory and field bioassays. Isobutanol was not attractive to B. oleae in either laboratory bioassay or field bioassay. Identifying yeast volatiles attractive to the olive fruit fly may lead to development of a more effective lure for detection, monitoring, and possibly control of B. oleae.
Characterization of the aroma profile and key odorants of the Spanish PDO wine vinegars.[Pubmed:31855771]
Food Chem. 2020 May 1;311:126012.
The aroma profiles of Spanish wine vinegars with Protected Designation of Origin (PDO) were described and compared for the first time by gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor-active values (OAVs) and quantitative descriptive analysis (QDA). Vinagre de Jerez Reserva (JRE) showed higher percentage of 'grassy-vegetal' impact odorants, while 'spicy' compounds highlighted the Pedro Ximenez category (JPX). Vinagre de Montilla-Moriles Reserva (MRE) had 'buttery-lactic' impact odorants, while 'empyreumatic' and 'sweet' aromas stood out for Pedro Ximenez category (MPX). Vinagre de Condado de Huelva Reserva (CRE) showed a stronger percentage of 'chemical' impact odorants. The key odorants were ethyl propionate, ethyl octanoate, propanoic acid and 4-ethylphenol for JRE, diacetyl and methional/furfural for JPX, acetoin for MRE, ethyl phenylacetate and vanillin for MPX and acetaldehyde diethyl acetal, Isobutyl acetate, ethyl isovalerate and guaiacol for CRE. A good relation among the impact odorants and the sensory descriptors was observed.
Single mutation at a highly conserved region of chloramphenicol acetyltransferase enables isobutyl acetate production directly from cellulose by Clostridium thermocellum at elevated temperatures.[Pubmed:31636704]
Biotechnol Biofuels. 2019 Oct 15;12:245.
Background: Esters are versatile chemicals and potential drop-in biofuels. To develop a sustainable production platform, microbial ester biosynthesis using alcohol acetyltransferases (AATs) has been studied for decades. Volatility of esters endows high-temperature fermentation with advantageous downstream product separation. However, due to the limited thermostability of AATs known, the ester biosynthesis has largely relied on use of mesophilic microbes. Therefore, developing thermostable AATs is important for ester production directly from lignocellulosic biomass by the thermophilic consolidated bioprocessing (CBP) microbes, e.g., Clostridium thermocellum. Results: In this study, we engineered a thermostable chloramphenicol acetyltransferase from Staphylococcus aureus (CATSa) for enhanced Isobutyl acetate production at elevated temperatures. We first analyzed the broad alcohol substrate range of CATSa. Then, we targeted a highly conserved region in the binding pocket of CATSa for mutagenesis. The mutagenesis revealed that F97W significantly increased conversion of isobutanol to Isobutyl acetate. Using CATSa F97W, we demonstrated direct conversion of cellulose into Isobutyl acetate by an engineered C. thermocellum at elevated temperatures. Conclusions: This study highlights that CAT is a potential thermostable AAT that can be harnessed to develop the thermophilic CBP microbial platform for biosynthesis of designer bioesters directly from lignocellulosic biomass.
Laboratory and Field Evaluation of Host-Related Foraging Odor-Cue Combinations to Attract Drosophila suzukii (Diptera: Drosophilidae).[Pubmed:31429468]
J Econ Entomol. 2019 Dec 9;112(6):2850-2860.
The invasive spotted-wing drosophila, Drosophila suzukii (Matsumura), is a major pest of soft-skinned fruits. Since its introduction into North America and Europe, significant progress has been made in understanding the volatile cues used by this fly during food, oviposition site, and mate finding. Despite this progress, commercially available lures are non-selective. Here, we tested two Hanseniaspora uvarum (Niehaus) yeast compounds (isoamyl acetate and Isobutyl acetate) and a leaf compound beta-cyclocitral alone and in combination with a blend of four fermentation compounds ('Fermentation lure': acetic acid, ethanol, methionol, and acetoin) to improve D. suzukii attraction and selectivity. In laboratory assays, males and females were attracted to all seven individual compounds, although in electrophysiological assays, their antennae exhibited a dose-dependent response to only four of these compounds. In two-choice cage studies, the Fermentation lure was more attractive to D. suzukii than water controls, whereas beta-cyclocitral and the mixture of isoamyl acetate and Isobutyl acetate were not attractive in this larger-cage study. Moreover, adding the two-component H. uvarum compound blend to the Fermentation lure reduced D. suzukii attraction to the Fermentation blend. When these experiments were repeated in blueberry, raspberry, blackberry, and cherry orchards across several states in the United States over 2 yr, similar outcomes were observed: beta-cyclocitral or the mixture of the H. uvarum blend did not improve the attractiveness of the Fermentation lure or its selectivity. This study demonstrates that cues from different sources may interfere with each other and reduce D. suzukii attraction to otherwise attractive odor combinations.
Indigenous Yeast Interactions in Dual-Starter Fermentations May Improve the Varietal Expression of Moschofilero Wine.[Pubmed:31402907]
Front Microbiol. 2019 Jul 26;10:1712.
Multi-starter wine fermentations employing non-Saccharomyces (NS) yeasts are becoming an emerging trend in winemaking. It is therefore important to determine the impacts of different NS strains in the wine phenotype and in particular the aroma outputs in different inoculation schemes and fermentation conditions. Here, two native NS yeasts, Lachancea thermotolerans LtMM7 and Hanseniaspora uvarum HuMM19, were assessed for their ability to improve the quality of Moschofilero, a Greek aromatic white wine. The NS strains were initially examined in laboratory scale fermentations in mixed inoculations with ScMM23, a native Saccharomyces cerevisiae strain. LtMM7 was selected to be further evaluated in pilot scale fermentations. Five different inoculation schemes were considered: single inoculation of ScMM23 (IS), simultaneous inoculation of ScMM23 with HuMM19 (SMH) or LtMM7 (SML), and sequential inoculation of HuMM19 (SQH) or LtMM7 (SQL) followed by ScMM23. At laboratory scale fermentations, the chemical profiles were largely affected by both the NS species and the inoculation scheme applied. The sequential inoculation using HuMM19 produced the most divergent wine phenotype. However, HuMM19 caused significant increases in acetic acid and ethyl acetate levels that impeded its use in pilot scale trials. LtMM7 significantly affected the chemical profiles of wines produced at the winery, especially in the sequential inoculation scheme. Importantly, LtMM7 significantly increased the levels of acetate esters or ethyl esters, depending on the inoculation method applied. In particular, acetate esters like Isobutyl acetate, hexyl acetate, and 2-phenylethyl acetate, which all impart fruity or floral aromas, were significantly increased in SQL. On the other hand, higher levels of total ethyl esters were associated with SML. The most striking differences were observed in the levels of fruit-impair esters like ethyl decanoate, 3-methylbutyl octanoate, and isoamyl hexanoate. This is the first study to report a significant increase in the ethyl ester fraction by L. thermotolerans. Interestingly, L. thermotolerans in SQL also increased the concentrations of damascenone and geraniol, the major teprenic compound of Moschofilero, which are associated with several typical floral and fruity aromas of the variety. Present results show that L. thermotolerans may enhance the varietal character and increase the chemical complexity of Moschofilero wines.
RIFM fragrance ingredient safety assessment, isobutyl propionate, CAS Registry Number 540-42-1.[Pubmed:31233870]
Food Chem Toxicol. 2019 Aug;130 Suppl 1:110607.
The existing information supports the use of this material as described in this safety assessment. Isobutyl propionate was evaluated for genotoxicity, repeated dose toxicity, reproductive toxicity, local respiratory toxicity, phototoxicity/photoallergenicity, skin sensitization, and environmental safety. Data from read-across analog Isobutyl acetate (CAS # 110-19-0) show that isobutyl propionate is not expected to be genotoxic. Data from read-across analog isoamyl acetate (CAS # 123-92-2) show that there are no safety concerns for isobutyl propionate for skin sensitization under the current declared levels of use. The repeated dose and reproductive endpoints were evaluated using the TTC for a Cramer Class I material, and the exposure to isobutyl propionate is below the TTC (0.03mg/kg/day and 0.03mg/kg/day, respectively). For the local respiratory endpoint, a calculated MOE >100 was provided by read-across analog butyl acetate (CAS # 123-86-4). The phototoxicity/photoallergenicity endpoints were evaluated based on UV spectra; isobutyl propionate is not expected to be phototoxic/photoallergenic. The environmental endpoints were evaluated; isobutyl propionate is not PBT as per the IFRA Environmental Standards. For the risk assessment, isobutyl propionate was not able to be risk screened as there were no reported volumes of use for North America or Europe in the 2015 IFRA Survey.
DPD Parameters Estimation for Simultaneously Simulating Water-Oil Interfaces and Aqueous Nonionic Surfactants.[Pubmed:30376315]
J Chem Theory Comput. 2018 Dec 11;14(12):6460-6471.
The outcome of a coarse-grained simulation within the dissipative particle dynamics framework strongly depends on the choice of the repulsive parameter between different species. Different methodologies have been used in the literature to determine these parameters toward reproducing selected experimental system properties. In this work, a systematic investigation on possible procedures for estimating the simulation parameters is conducted. We compare methods based on the Hildebrand and the Hansen solubility parameter theories, mapped into the Flory-Huggins model. We find that using the Hansen solubility parameters it is possible to achieve a high degree of coarse graining, with parameters that yield realistic values for the interfacial tension. The procedure was first applied to the water/benzene system and then validated for water/ n-octane, water/1,1-dichloroethane, water/methyl cyclohexane, and water/Isobutyl acetate. In all these cases, the experimental interfacial tension could be reproduced by adjusting a single correction factor. In the case of the water-benzene system, the dissipative particle dynamics parameters derived using our approach were able to simultaneously describe both the interfacial tension and micellar properties of aqueous nonionic surfactants representative of the octyl poly(ethylene oxide) C8H17O(C2H4O)mH family. We show how the parameters can be used, within the dissipative particle dynamics framework, to simulate the water/oil interface in the presence of surfactants at varying concentrations. The results show, as expected, that as the surfactant concentration increases, the interfacial tension decreases, and micelles form in bulk water.


