IsoboldineCAS# 3019-51-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3019-51-0 | SDF | Download SDF |
| PubChem ID | 133323 | Appearance | Brown powder |
| Formula | C19H21NO4 | M.Wt | 327.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
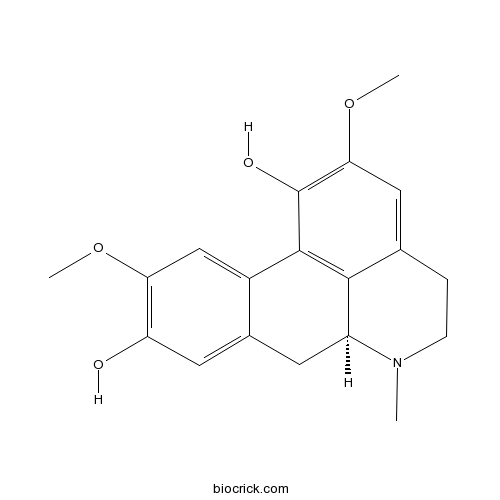
| Chemical Name | (6aS)-2,10-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-1,9-diol | ||
| SMILES | CN1CCC2=CC(=C(C3=C2C1CC4=CC(=C(C=C43)OC)O)O)OC | ||
| Standard InChIKey | LINHZVMHXABQLB-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C19H21NO4/c1-20-5-4-10-8-16(24-3)19(22)18-12-9-15(23-2)14(21)7-11(12)6-13(20)17(10)18/h7-9,13,21-22H,4-6H2,1-3H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isoboldine Dilution Calculator

Isoboldine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0544 mL | 15.2718 mL | 30.5437 mL | 61.0874 mL | 76.3592 mL |
| 5 mM | 0.6109 mL | 3.0544 mL | 6.1087 mL | 12.2175 mL | 15.2718 mL |
| 10 mM | 0.3054 mL | 1.5272 mL | 3.0544 mL | 6.1087 mL | 7.6359 mL |
| 50 mM | 0.0611 mL | 0.3054 mL | 0.6109 mL | 1.2217 mL | 1.5272 mL |
| 100 mM | 0.0305 mL | 0.1527 mL | 0.3054 mL | 0.6109 mL | 0.7636 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydrojuncuenin B
Catalog No.:BCN9294
CAS No.:1161681-28-2
- Latinone
Catalog No.:BCN9293
CAS No.:79157-36-1
- Juncutol
Catalog No.:BCN9292
CAS No.:1021950-14-0
- Lycojaponicuminol C
Catalog No.:BCN9291
CAS No.:1651839-34-7
- 6'-O-Galloylsalidroside
Catalog No.:BCN9290
CAS No.:83013-86-9
- Rubanthrone A
Catalog No.:BCN9289
CAS No.:441764-20-1
- Browniine
Catalog No.:BCN9288
CAS No.:5140-42-1
- Oregonin
Catalog No.:BCN9287
CAS No.:55303-93-0
- Palmitone
Catalog No.:BCN9286
CAS No.:502-73-8
- Phoyunbene C
Catalog No.:BCN9285
CAS No.:886747-63-3
- 1-O-p-Coumaroylglycerol
Catalog No.:BCN9284
CAS No.:106055-11-2
- N1,N5,N10-Tri-p-coumaroylspermidine
Catalog No.:BCN9283
CAS No.:131086-78-7
- (+)-Maackiain
Catalog No.:BCN9296
CAS No.:23513-53-3
- 16-Oxolycoclavanol
Catalog No.:BCN9297
CAS No.:53800-21-8
- Changweikang aldehyde
Catalog No.:BCN9298
CAS No.:2119605-13-7
- Ugandenial A
Catalog No.:BCN9299
CAS No.:1175880-15-5
- Pholidotol C
Catalog No.:BCN9300
CAS No.:1013909-91-5
- 1,6-Dimethyl-4,5-dihydropyrene-2,7-diol
Catalog No.:BCN9301
CAS No.:2255331-28-1
- ent-13,16β,17-Trihydroxykauran-19-oic acid
Catalog No.:BCN9302
CAS No.:142543-30-4
- n-Butyl β-D-fructofuranoside
Catalog No.:BCN9303
CAS No.:80971-60-4
- Taxilluside A
Catalog No.:BCN9304
CAS No.:1661914-46-0
- 3,5,3'-Trihydroxybibenzyl
Catalog No.:BCN9305
CAS No.:86630-23-1
- Simulanol
Catalog No.:BCN9306
CAS No.:500574-38-9
- 3,5,3'-Trihydroxystilbene
Catalog No.:BCN9307
CAS No.:150258-84-7
Anti-inflammatory principles from Lindera aggregata.[Pubmed:32359855]
Bioorg Med Chem Lett. 2020 Jul 1;30(13):127224.
Four new sesquiterpenes (1-4), one new alkaloid (5), and one new benzenoid glycoside (6) were characterized from Lindera aggregata, and their structures were elucidated according to their spectrometric analytical data. Among these isolates, 3 and 4 were constructed as possessing unprecedented carbon skeletons from the natural source. Some of these purified constituents were examined for their anti-inflammatory bioactivity. Among the tested compounds, linderaggredin C (3), (+)-N-methyllaurotetanine, and (+)-Isoboldine displayed the significant inhibition of superoxide anion generation in human neutrophils with IC50 values of 7.45 +/- 0.74, 8.36 +/- 0.11, and 5.81 +/- 0.59 muM, respectively.
[Chemical constituents in chloroform fraction of Houttuynia cordata].[Pubmed:30989951]
Zhongguo Zhong Yao Za Zhi. 2019 Jan;44(2):314-318.
Nine compounds were isolated from chloroform fraction of Houttuynia cordata,and the isolates were identified as follows:( S)-5,6,6 a,7-tetrahydro-2,10-dimethoxy-4 H-dibenzo [DE,G] quinoline-1,9-diol( 1),( +)-Isoboldine beta-N-oxide( 2),liriotulipiferine( 3),telitoxinone( 4),Isoboldine( 5),(-)-clovane-2beta,9alpha-diol( 6),benzoic acid( 7),acantrifoside E( 8),and dibutyl phthalate( 9). Among them,compound 1 was new,and compounds 2-9 were reported from this species for the first time.
Direct infusion ESI-IT-MSn alkaloid profile and isolation of tetrahydroharman and other alkaloids from Bocageopsis pleiosperma maas (Annonaceae).[Pubmed:26108161]
Phytochem Anal. 2015 Sep-Oct;26(5):339-45.
INTRODUCTION: The Annonaceae family is known as a promising abundant source of secondary metabolites, especially annonaceous acetogenins, terpenoids and isoquinoline-derived alkaloids. Although widely investigated from the phytochemical viewpoint, this family still presents some largely unexplored genera, e.g. the Bocageopsis. OBJECTIVE: To investigate the alkaloid content of Bocageopsis pleiosperma Maas using direct infusion electrospray ionisation ion trap tandem mass spectrometry (ESI-IT-MS(n)) analysis. METHODOLOGY: Dichloromethane extracts of aerial parts were subjected to acid-base partitioning to yield the alkaloidal fractions. These fractions were analysed by direct infusion into a (+)ESI-IT-MS(n) system. The alkaloidal fraction from the leaves was also obtained on a large scale and subjected to chromatographic separation. RESULTS: The tentative MS(n) -based identification of alkaloids in leaves, twigs and trunk bark showed that aporphine alkaloids were restricted to the leaves and twigs, tetrahydroprotoberberine alkaloids were only found in the twigs and trunk bark while benzylisoquinoline alkaloids were found in the leaves, twigs and trunk bark. Chromatographic separation of the leaf alkaloidal fraction yielded the aporphine alkaloids nornuciferine, asimilobine and Isoboldine, the beta-carboline alkaloid tetrahydroharman and some mixtures containing benzylisoquinoline and aporphine alkaloids, all described for the first time in the Bocageopsis genus. Furthermore, tetrahydroharman has not previously been reported in the Magnoliales order. CONCLUSION: Direct infusion ESI-IT-MS(n) analysis of alkaloids allowed fast recognition of alkaloidal classes previously reported in the Annonaceae family, aiding the chromatographic step and allowing a selective isolation of compounds previously not identified in the Bocageopsis genus.
Pharmacokinetics and metabolism study of isoboldine, a major bioactive component from Radix Linderae in male rats by UPLC-MS/MS.[Pubmed:26055342]
J Ethnopharmacol. 2015 Aug 2;171:154-60.
ETHNOPHARMACOLOGICAL RELEVANCE: Isoboldine is one of the major bioactive constituents in the total alkaloids from Radix Linderae (TARL) which could effectively alleviate inflammation and joints destruction in mouse collagen-induced arthritis. To better understand its pharmacological activities, we need to determine its pharmacokinetic and metabolic profiles. MATERIALS AND METHODS: In this study, a sensitive and simple UPLC-MS/MS method was developed and validated for determination of Isoboldine in rat plasma. Isoboldine in plasma was recovered by liquid-liquid extraction using 1 mL of methyl tert-butyl ether. Chromatographic separation was performed on a C18 column at 45 degrees C, with a gradient elution consisting of acetonitrile and water containing 0.1% (v/v) formic acid at a flow rate of 0.3 mL/min. The detection was performed on an electrospray triple-quadrupole MS/MS by positive ion multiple-reaction monitoring mode. This newly developed method was successfully applied to a pharmacokinetic study after oral and intravenous dosing in rats. For metabolites identification, Isoboldine was orally administered to rats and the metabolite in plasma, bile, urine and feces were characterized by the established UPLC-MS/MS method. RESULTS: Good linearity (r(2)>0.9956) was achieved in a concentration range of 4.8-2400 ng/mL with a lower limit of quantification of 4.8 ng/mL for Isoboldine. The intra- and inter-day precisions of the assay were 1.7-5.1% and 2.2-4.4% relative standard deviation with an accuracy of 91.3-102.3%. A total of five phase II metabolites in rat plasma, bile, urine and feces were characterized by comparing retention time in UPLC, and by molecular mass and fragmentation pattern of the metabolites by mass spectrometry with those of Isoboldine. CONCLUSION: Isoboldine has extremely low oral bioavailability due to the strong first-pass effect by the rats, and glucuronidation and sulfonation were involved in metabolic pathways of Isoboldine in rats. These results have paved the way for further clarifying therapeutic ingredients and provided new knowledge regarding pharmacokinetic features of this category of isoquinoline alkaloids.
[Alkaloids from roots and stems of Litsea cubeba].[Pubmed:25751947]
Zhongguo Zhong Yao Za Zhi. 2014 Oct;39(20):3964-8.
A phytochemical investigation on the roots and stems of Litsea cubeba led to the isolation of seven isoquinolone alkaloids. By spectroscopic analysis and comparison of their 1H and 13C-NMR data with those in literatures, these alkaloids were identified as (+)-norboldine (1), (+)-boldine (2), (+)-reticuline (3), (+)-laurotetanine (4), (+)-Isoboldine (5), (+)-N-methyl-laurotetanine (6), and berberine (7), respectively. Among them, 7 was isolated from the genus for the first time. The evaluation of these compounds showed weak anti-inflammatory activity against NO production in RAW 267.4 and BV-2 cells.
Plant germination and production of callus from the yellow hornpoppy (Glaucium flavum): the first stage of micropropagation.[Pubmed:25272947]
Pharmazie. 2014 Sep;69(9):715-20.
The yellow hornpoppy, Glaucium flavum Cr. (Fam. Papaveraceae) is a perennial herb, distributed in the Mediterranean region, including Egypt. The plant contains many benzyl isoquinoline alkaloids from the aporphine type such as glaucine, Isoboldine, 1-chelidonine, 1-norchelidonine and 3-O-methylarterenol, making it to display various medicinal activities including antitussive, anticancer, antioxidant, antimicrobial, antiviral, hypoglycemic, analgesic, antipyretic, bronchodilator and anti-inflammatory effects. The plant is now rare and endangered in the Egyptian flora due to urban sprawl. The present study looks into Glaucium flavum seeds' in vitro germination as well as the ability of the explants taken from the growing seedlings to form stable callus lines in order to enable micropropagation as a way to save the rare plant. The study also scans the production of different medicinally valuable alkaloids, particularly glaucine, in produced callus.
Rat CYP2D2, not 2D1, is functionally conserved with human CYP2D6 in endogenous morphine formation.[Pubmed:22641033]
FEBS Lett. 2012 Jun 21;586(13):1749-53.
The assumption that CYP2D1 is the corresponding rat cytochrome to human CYP2D6 has been revisited using recombinant proteins in direct enzyme assays. CYP2D1 and 2D2 were incubated with known CYP2D6 substrates, the three morphine precursors thebaine, codeine and (R)-reticuline. Mass spectrometric analysis showed that rat CYP2D2, not 2D1, catalyzed the 3-O-demethylation reaction of thebaine and codeine. In addition, CYP2D2 incubated with (R)-reticuline generated four products corytuberine, pallidine, salutaridine and Isoboldine while rat CYP2D1 was completely inactive. This intramolecular phenol-coupling reaction follows the same mechanism as observed for CYP2D6. Michaelis-Menten kinetic parameters revealed high catalytic efficiencies for rat CYP2D2. These findings suggest a critical evaluation of other commonly accepted, however untested, CYP2D1 substrates.
Anti-acetylcholinesterase, anti-alpha-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species.[Pubmed:22119096]
Fitoterapia. 2012 Mar;83(2):298-302.
Phytochemical investigation of Beilschmiedia alloiophylla has resulted in the isolation of one new alkaloid, 2-hydroxy-9-methoxyaporphine (1), and ten known natural products, laurotetanine (2), liriodenine (3), boldine (4), secoboldine (5), Isoboldine (6), asimilobine (7), oreobeiline (8), 6-epioreobeiline (9), beta-amyrone (10), and (S)-3-methoxynordomesticine (11). Chemical studies on the bark of B. kunstleri afforded compounds 2 and 4 along with one bisbenzylisoquinoline alkaloid, N-dimethylphyllocryptine (12). Structures of compounds 1-12 were elucidated on the basis of spectroscopic methods. All of these isolates were evaluated for their anti-acetylcholinesterase (AChE), anti-alpha-glucosidase, anti-leishmanial and anti-fungal activities. Compounds 1-12 exhibited strong to moderate bioactivities in aforementioned bioassays.
Mammalian cytochrome P450 enzymes catalyze the phenol-coupling step in endogenous morphine biosynthesis.[Pubmed:19561069]
J Biol Chem. 2009 Sep 4;284(36):24425-31.
A cytochrome P450 (P450) enzyme in porcine liver that catalyzed the phenol-coupling reaction of the substrate (R)-reticuline to salutaridine was previously purified to homogeneity (Amann, T., Roos, P. H., Huh, H., and Zenk, M. H. (1995) Heterocycles 40, 425-440). This reaction was found to be catalyzed by human P450s 2D6 and 3A4 in the presence of (R)-reticuline and NADPH to yield not a single product, but rather (-)-Isoboldine, (-)-corytuberine, (+)-pallidine, and salutaridine, the para-ortho coupled established precursor of morphine in the poppy plant and most likely also in mammals. (S)-Reticuline, a substrate of both P450 enzymes, yielded the phenol-coupled alkaloids (+)-Isoboldine, (+)-corytuberine, (-)-pallidine, and sinoacutine; none of these serve as a morphine precursor. Catalytic efficiencies were similar for P450 2D6 and P450 3A4 in the presence of cytochrome b(5) with (R)-reticuline as substrate. The mechanism of phenol coupling is not yet established; however, we favor a single cycle of iron oxidation to yield salutaridine and the three other alkaloids from (R)-reticuline. The total yield of salutaridine formed can supply the 10 nm concentration of morphine found in human neuroblastoma cell cultures and in brain tissues of mice.
Antimicrobial evaluation of clerodane diterpenes from Polyalthia longifolia var. pendula.[Pubmed:19413108]
Nat Prod Commun. 2009 Mar;4(3):327-30.
Phytochemical investigation of the ethanolic extract of leaves of Polyalthia longifolia var. pendula has led to the isolation of seven clerodane diterpenoids and five alkaloids. (-)-14, 15-bisnor-3, 11E-kolavadien-13-one (1), (-)-16-oxocleroda-3,13(14)E-dien-15-oic acid (2), (-)-16alpha-hydroxycleroda-3,13 (14)Z-dien-15,16-olide (3), (+)-(4-->2)-abeo-16(R/S)-2, 13Z-kolavadien-15, 16-olide-3-al (4), (-)-3beta, 16beta-dihydroxycleroda-4(18), 13(14)Z-dien-15,16-olide (5), (-)-3, 12E-kolavadien-15-oic acid-16-al (6), (-)-labd-13E-en-8-ol-15-oic acid (7), liriodenine (8), (-)-anonaine (9), (+)-Isoboldine (10), (-)-asimilobine (11) and hordenine (12) have been isolated. This is the first report of 1, 6 and 10 from this plant species while 12 is reported for first time from this genus. Clerodane derivatives 1-7 were evaluated for their antimicrobial activity. Diterpene 3 was found to be most potent agent with MIC value of 6.25 microg/mL against Staphylococcus aureus and Sporothrix schenckii.
Antimicrobially active isoquinoline alkaloids from Litsea cubeba.[Pubmed:18991207]
Planta Med. 2009 Jan;75(1):76-9.
Bioassay-guided fractionation of the alkaloidal extract of the aerial part of Litsea cubeba led to the isolation of two new isoquinoline alkaloids, (+)- N-(methoxycarbonyl)-N-norboldine (1) and (+)-Isoboldine beta-N-oxide (2), together with 11 known analogues (3-13). Their structures were established by extensive spectroscopic techniques and by comparing spectroscopic data with those in the literature. Compounds 1 and 4 showed antimicrobial activities. This is the first report on the presence of compounds 1, 2, 6, 8, 9, 11, and 12 in this plant and on the antimicrobial activities of 1 and 4. The bioactivities of isoquinoline alkaloids are also at least partly responsible for the pharmacological function of the folk medicinal plant Litsea cubeba.
Online structural elucidation of alkaloids and other constituents in crude extracts and cultured cells of Nandina domestica by combination of LC-MS/MS, LC-NMR, and LC-CD analyses.[Pubmed:18671433]
J Nat Prod. 2008 Aug;71(8):1376-85.
The combination of NMR, MS, and CD data permitted the structural elucidation including the absolute configuration of the known alkaloids and unknown components in the extract matrix solution of Nandina domestica without isolation and sample purification prior to the coupling experiments. Unstable natural stereoisomers were identified by LC-NMR and LC-MS. Five known alkaloids, (S)-Isoboldine, (S)-domesticine, (S)-nantenine, sinoacutine, and menispermine, were identified from N. domestica. O-Methylpallidine and (E, E)-, (E, Z)-, and (Z, Z)-terrestribisamide were also characterized for the first time from this plant. Known jatrorrhizine, palmatine, and berberine and unknown (R)-carnegine and (E, E)-, (E, Z)-, and (Z, Z)-terrestribisamide were identified in the callus of N. domestica.
Minor alkaloids from Guatteria dumetorum with antileishmanial activity.[Pubmed:16534735]
Planta Med. 2006 Feb;72(3):270-2.
Nine known alkaloids [(+)-isodomesticine (1), (+)-norisodomesticine (2), (+)-nantenine ( 3), (+)-neolitsine (4), (+)-lirioferine (5), (+)-N-methyllaurotetanine (6), (+)-norlirioferine (7), (+)-Isoboldine (8) and (+)-reticuline (9)] were isolated from young leaves of Guatteria dumetorum. Their structures were confirmed by NMR, mass and UV spectral analysis and by comparison to literature data. The growth inhibitory activity of each alkaloid was determined against the parasite Leishmania mexicana. Compounds 1-4 all showed significant activity whereby potency increased when a methylenedioxy functionality was present, especially at the 1,2-positions.
[Alkaloids from the root of Lindera angustifolia].[Pubmed:16408812]
Yao Xue Xue Bao. 2005 Oct;40(10):931-4.
AIM: To study the alkaloid constituents of the root of Lindera angustifolia Cheng. METHODS: The constituents were isolated and purified by column chromatography and the structures were characterized by spectral analysis. RESULTS: Seven aporphine alkaloids, laurotetanine (I), N-methyllaurotetanine (II), boldine (III), Isoboldine (IV), norboldine (V), N-ethoxycarbonyllaurotetanine (VII) and a quaternary isoquinoline alkaloid, magnocurarine (VI), were isolated and identified. The structure of VII was further identified by semi-synthesis with I as starting material. CONCLUSION: All compounds were obtained from this plant for the first time and compound VII was found as a naturally occurring compound for the first time.
Geographic distribution of three alkaloid chemotypes of Croton lechleri.[Pubmed:12088421]
J Nat Prod. 2002 Jun;65(6):814-9.
Three known alkaloids, Isoboldine (2), norIsoboldine (1), and magnoflorine (8), have been isolated for the first time from Croton lechleri, a source of the wound healing latex "sangre de grado". An HPLC system was developed, and a large number of latex and leaf samples of C. lechleri from 22 sites in northern Peru and Ecuador were analyzed to gain an understanding of the natural variation in alkaloid content for the species. Up to six alkaloids were found to occur in the leaves including, in addition to those listed above, thaliporphine (3), glaucine (4), and taspine (9), whereas the latex contained only 9. Taspine (9) is the component that has been previously found to be responsible for the wound healing activity of C. lechleri latex, and its mean concentration throughout the range examined was found to be 9% of the latex by dry weight. In addition, three chemotypes are defined based on the alkaloid content of the leaves, and the geographic distribution of these chemotypes is discussed along with a quantitative analysis of the alkaloid content as a function of chemotype.


