IsobellidifolinCAS# 552-00-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 552-00-1 | SDF | Download SDF |
| PubChem ID | 5322042.0 | Appearance | Powder |
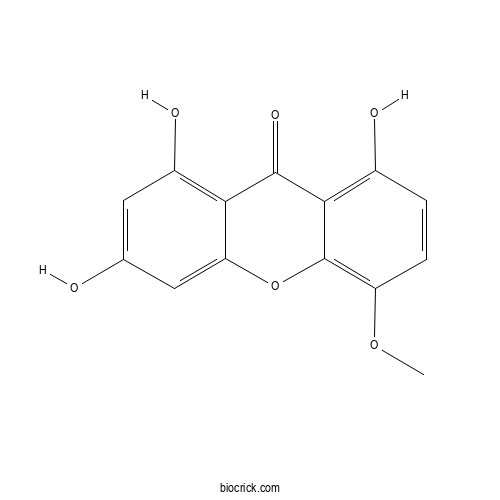
| Formula | C14H10O6 | M.Wt | 274.23 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,3,8-trihydroxy-5-methoxyxanthen-9-one | ||
| SMILES | COC1=C2C(=C(C=C1)O)C(=O)C3=C(C=C(C=C3O2)O)O | ||
| Standard InChIKey | QNJCJDZKEAVOBJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O6/c1-19-9-3-2-7(16)12-13(18)11-8(17)4-6(15)5-10(11)20-14(9)12/h2-5,15-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isobellidifolin Dilution Calculator

Isobellidifolin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6466 mL | 18.2329 mL | 36.4657 mL | 72.9315 mL | 91.1644 mL |
| 5 mM | 0.7293 mL | 3.6466 mL | 7.2931 mL | 14.5863 mL | 18.2329 mL |
| 10 mM | 0.3647 mL | 1.8233 mL | 3.6466 mL | 7.2931 mL | 9.1164 mL |
| 50 mM | 0.0729 mL | 0.3647 mL | 0.7293 mL | 1.4586 mL | 1.8233 mL |
| 100 mM | 0.0365 mL | 0.1823 mL | 0.3647 mL | 0.7293 mL | 0.9116 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Napelline
Catalog No.:BCX1285
CAS No.:5008-52-6
- Morusignin L
Catalog No.:BCX1284
CAS No.:149733-95-9
- 5-Hydroxy-3,7-dimethoxyflavone
Catalog No.:BCX1283
CAS No.:70786-48-0
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Hispidol
Catalog No.:BCX1276
CAS No.:5786-54-9
- 2',4,4',6'-Tetramethoxychalcone
Catalog No.:BCX1275
CAS No.:94103-36-3
- 2'-O-Methylphloretin
Catalog No.:BCX1274
CAS No.:111316-17-7
- Dihydrocatalpol
Catalog No.:BCX1287
CAS No.:6736-86-3
- Ajugose
Catalog No.:BCX1288
CAS No.:512-72-1
- Limocitrin 3-O-β-D-glucopyranoside
Catalog No.:BCX1289
CAS No.:38836-51-0
- Wistin
Catalog No.:BCX1290
CAS No.:19046-26-5
- Physcion-8-O-β-gentiobioside
Catalog No.:BCX1291
CAS No.:84268-38-2
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
- Hypoglaunine D
Catalog No.:BCX1296
CAS No.:220751-00-8
- Kanshone C
Catalog No.:BCX1297
CAS No.:117634-64-7
- Lyciumin A
Catalog No.:BCX1298
CAS No.:125708-06-7
Chemical Composition, Antioxidant and Anticholinesterase Activities of Gentianella azurea from Russian Federation.[Pubmed:30549824]
Nat Prod Commun. 2017 Jan;12(1):55-56.
Phytochemical study of Gentianella azurea (Bunge) Holub (Gentianaceae) collected in Buryatia Republic (Russian Federation) resulted in the isolation of twenty-one compounds including bellidifolin, bellidin, Isobellidifolin, norswertianolin, Isobellidifolin-8-O-beta-D-glucopyranoside, orientin, cynaroside, .cosmosiin, apigenin, 4'-O-caffeoylswertiamarin, swertiamarin-6'-O-beta-D-glucopyranoside and sweroside, firstly detected in this species. The extracts and individual compounds were shown to possess antioxidant and anticholinesterase potential.
Antimicrobial and antioxidant activities of Gentianella multicaulis collected on the Andean Slopes of San Juan Province, Argentina.[Pubmed:22486039]
Z Naturforsch C J Biosci. 2012 Jan-Feb;67(1-2):29-38.
The infusion of the aerial parts of Gentianella multicaulis (Gillies ex Griseb.) Fabris (Gentianaceae), locally known as 'nencia', is used in San Juan Province, Argentina, as stomachic and as a bitter tonic against digestive and liver problems. The bioassay-guided isolation of G. multicaulis extracts and structural elucidation of the main compounds responsible for the antifungal and free radical scavenging activities were performed. The extracts had strong free radical scavenging effects in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (45-93% at 10 microg/mL) and ferric-reducing antioxidant power (FRAP) assay at 200 microg/mL. Demethylbellidifolin (4) had high antioxidant activity in the DPPH and FRAP assay. The dermatophytes Microsporum gypseum, Trichophyton mentagrophytes, and T. rubrum were moderately inhibited by the different extracts (MIC values of 125-250 microg/mL). Demethylbellidifolin (4), bellidifolin (5), and Isobellidifolin (6) showed an antifungal effect (MIC values of 50 microg/mL), while swerchirin (3) was less active with a MIC value of 100 microg/mL. In addition, oleanolic acid (1) and ursolic acid (2) were also isolated. These findings demonstrate that Gentianella multicaulis collected in the mountains of the Province of San Juan, Argentina, is an important source of compounds with antifungal and antioxidant activities.
Activity-guided isolation of antioxidant xanthones from Swertia chirayita (Roxb.) H. Karsten (Gentianaceae).[Pubmed:21985644]
Nat Prod Res. 2012;26(18):1682-6.
An activity-guided isolation and purification process was used to identify the DPPH (l,l-diphenyl-2-picrylhydrazyl) radical-scavenging components of Swertia chirayita. A dry, whole plant of S. chirayita was extracted with different solvents and tested for its DPPH radical-scavenging activity. The acetone : water (8 : 2) extract showed the highest total phenolic content (TPC) and DPPH radical-scavenging activity, which was column chromatographed to obtain decussatin, swertianin, bellidifolin, Isobellidifolin, amarogentin, swertianolin and mangiferin as active components. Good correlation was observed between TPC and DPPH scavenging activity among the extracts. The unique structure of xanthones, including the catecholic moiety and the completely conjugated system, enables them to be promising antioxidants.
Two new xanthone diglycosides from Swertia longifolia Boiss.[Pubmed:17127518]
Nat Prod Res. 2006 Nov;20(13):1251-7.
From aerial parts of Swertia longifolia Boiss., which grows in the north of Iran, five xanthones, two of which in diglycosidic form, were isolated. The structures were confirmed by means of their spectral data as Isobellidifolin, bellidin, gentisein, 1,5-dihydroxy-3-methoxy-6-O-primeverosyl xanthone, and 8-hydroxy-3,5-dimethoxy-1-O-primeverosyl xanthone, the latter two of which were new derivatives in the plant kingdom.
Xanthones from Swertia punctata.[Pubmed:12377236]
Phytochemistry. 2002 Oct;61(4):415-20.
Isolation of 1-O-primeverosyl-3,8-dihydroxy-5-methoxyxanthone and 1-O-gentiobiosyl-3,7-dimethoxy-8-hydroxyxanthone, along with five known xanthones, Isobellidifolin, methylbellidifolin, isoswertianin, methylswertianin and norswertianin-1-O-beta-D-glucoside, from the roots of Swertia punctata is reported. In the aerial parts four xanthones, bellidifolin, methylbellidifolin, swertianolin and mangiferin, and flavone-C-glucoside, isoorientin were identified. The chemotaxonomic and pharmacological significance of these results is discussed.


