(+)-IsoajmalineCAS# 6989-79-3 |
- Ajmaline
Catalog No.:BCN3867
CAS No.:4360-12-7
Quality Control & MSDS
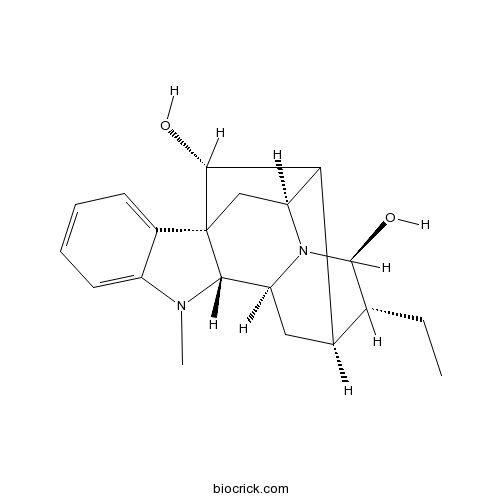
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6989-79-3 | SDF | Download SDF |
| PubChem ID | 251572 | Appearance | Powder |
| Formula | C20H26N2O2 | M.Wt | 326.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,9R,10S,12S,13R,14S,16S,18R)-13-ethyl-8-methyl-8,15-diazahexacyclo[14.2.1.01,9.02,7.010,15.012,17]nonadeca-2,4,6-triene-14,18-diol | ||
| SMILES | CCC1C2CC3C4C5(CC(C2C5O)N3C1O)C6=CC=CC=C6N4C | ||
| Standard InChIKey | CJDRUOGAGYHKKD-AVQYEUALSA-N | ||
| Standard InChI | InChI=1S/C20H26N2O2/c1-3-10-11-8-14-17-20(12-6-4-5-7-13(12)21(17)2)9-15(16(11)18(20)23)22(14)19(10)24/h4-7,10-11,14-19,23-24H,3,8-9H2,1-2H3/t10-,11-,14+,15+,16?,17+,18-,19+,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Helvetica Chimica Acta, 1981, 64(1):90-96.Die absolute Konfiguration von Macrolin, einem Abbauprodukt des Alkaloides Villalstonin 179. Mitteilung über organische Naturstoffe.[Reference: WebLink]
|

(+)-Isoajmaline Dilution Calculator

(+)-Isoajmaline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0637 mL | 15.3186 mL | 30.6373 mL | 61.2745 mL | 76.5931 mL |
| 5 mM | 0.6127 mL | 3.0637 mL | 6.1275 mL | 12.2549 mL | 15.3186 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0637 mL | 6.1275 mL | 7.6593 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6127 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6127 mL | 0.7659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Evodine
Catalog No.:BCN2630
CAS No.:6989-38-4
- Bayogenin
Catalog No.:BCN2458
CAS No.:6989-24-8
- Atractylone
Catalog No.:BCN3048
CAS No.:6989-21-5
- Pseudoginsenoside F11
Catalog No.:BCN1062
CAS No.:69884-00-0
- Petunidin-3-O-glucoside chloride
Catalog No.:BCN3025
CAS No.:6988-81-4
- Boc-Glu(OtBu)-ONp
Catalog No.:BCC3393
CAS No.:69876-58-0
- Bourjotinolone A
Catalog No.:BCN4259
CAS No.:6985-35-9
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- Noradrenaline Bitartrate
Catalog No.:BCC8343
CAS No.:51-40-1
- Agarotetrol
Catalog No.:BCN6763
CAS No.:69809-22-9
- 2,7-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one
Catalog No.:BCN1374
CAS No.:69804-59-7
- Swertiajaponin
Catalog No.:BCN2791
CAS No.:6980-25-2
- Rhapontisterone B
Catalog No.:BCN2664
CAS No.:698975-64-3
- Immethridine dihydrobromide
Catalog No.:BCC7328
CAS No.:699020-93-4
- Cinalbicol
Catalog No.:BCN7464
CAS No.:69904-85-4
- Cyanopindolol hemifumarate
Catalog No.:BCC6880
CAS No.:69906-86-1
- Swertisin
Catalog No.:BCN2762
CAS No.:6991-10-2
- 20(S),24(R)-Ocotillol
Catalog No.:BCN3891
CAS No.:69926-31-4
- Isocostic acid
Catalog No.:BCN4260
CAS No.:69978-82-1
- Triflurdine (Viroptic)
Catalog No.:BCC3873
CAS No.:70-00-8
- L-Glutathione Reduced
Catalog No.:BCC8030
CAS No.:70-18-8
- H-Asn-OH
Catalog No.:BCC2875
CAS No.:70-47-3
- 1-(4-Hydroxyphenyl)propan-1-one
Catalog No.:BCN4597
CAS No.:70-70-2
- H-Tyr(3-I)-OH
Catalog No.:BCC3265
CAS No.:70-78-0
A New Ajmaline-type Alkaloid from the Roots of Rauvolfia serpentina.[Pubmed:30520580]
Nat Prod Commun. 2017 Apr;12(4):495-498.
A new ajmaline-type alkaloid, 21-Omicron-methylisoajmaline (1), together with twenty-one known compounds, a mixture of beta-sitosterol (2) and stigmasterol (3), reserpinine (4); tetrahydroalstonine (5), reserpine (6), venoterpine (7), yohimbine (8), 6'-O-(3,4,5-trimethoxybenzoyl)glomeratose A (9), (+)-Isoajmaline (10), 3-epi-alpha-yohimbine (11), methyl 3,4,5-trimethoxy-trans-cinnamate (12), a mixture of beta-sitosterol 3-Omicron-beta-D-glucopyranoside (13) and stigmasterol 3-Omicron-beta-D- glucopyranoside (14), rescidine (15), 7-deoxyloganic acid (16), ajmaline (17), suaveoline (18), (+)-tetraphyllicine (19), loganic acid (20), 3-hydroxysarpagine (21), and sarpagine (22), were isolated from the roots of Rauvolla serpentina. Their structures were elucidated by spectroscopic data analysis and comparison with literature data. Compounds 11, 12 and 15 were for the first time identified from the genus Rauvolfla and 5, 7, 11, 12, 15, 18 and 22 were found from R. sepentina for the first time. Compound 11 showed moderate anticholinesterase activity with IC(5)(0) value of 15.58 muM, whereas 6 exhibited strong vasorelaxant activity with the EC(5)(0) value of 0.05 muM.
A 1H- and 13C-NMR study of seven ajmaline-type alkaloids.[Pubmed:17252506]
Planta Med. 1996 Dec;62(6):577-9.
1H- and 13C-NMR spectral data are presented for ajmaline (1), 17-O-acetylajmaline (2), (+)-Isoajmaline (3), isosandwichine (4), rauflorine (5), vincamajine (6), and vincamedine (7). Some corrections to the previously reported assignments of compound 6 (1H-NMR) and compounds 3, 4 and 7 (13C-NMR) have been made. The 13C-NMR chemical shifts for rauflorine 5 are presented for the first time.
Monitoring of ajmaline in plasma with high-performance liquid chromatography.[Pubmed:1517305]
J Chromatogr. 1992 Mar 13;575(1):87-91.
A rapid, reliable and sensitive assay for routine determination of ajmaline in plasma by high-performance liquid chromatography with fluorimetric detection is presented. A low limit of detection in plasma (less than 1 ng/ml ajmaline) could be achieved by the extraction of plasma samples and the use of fluorimetric detection. Deproteinization of the plasma sample instead of extraction, or the use of an ultraviolet detector, yielded a higher limit of detection (less than 50 ng/ml). Two different eluents were studied. Eluent 1 allowed clear separation of ajmaline from (+)-Isoajmaline and sandwicine, but did not separate isoajamaline from sandwicine. With eluent 2, separation of (+)-Isoajmaline and sandwicine was achieved, but separation of ajmaline from sandwicine was less optimal than with eluent 1. Therefore, eluent 1 was used for further clinical studies. No interference was observed from therapeutic doses of other commonly co-administered drugs, such as acetylsalicylic acid, digoxin, digitoxin, ranitidine, dopamine, dobutamine, furosemide, captopril or glycerol trinitrate. In addition, the chemical stability of ajmaline and a possible rearrangement of ajmaline to its stereoisomers (+)-Isoajmaline and sandwicine was studied in vivo and in vitro. Ajmaline proved to be unusually stable under both in vivo and in vitro conditions.


