Indacaterol MaleateCAS# 753498-25-8 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 753498-25-8 | SDF | Download SDF |
| PubChem ID | 9827599 | Appearance | Powder |
| Formula | C28H32N2O7 | M.Wt | 508.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
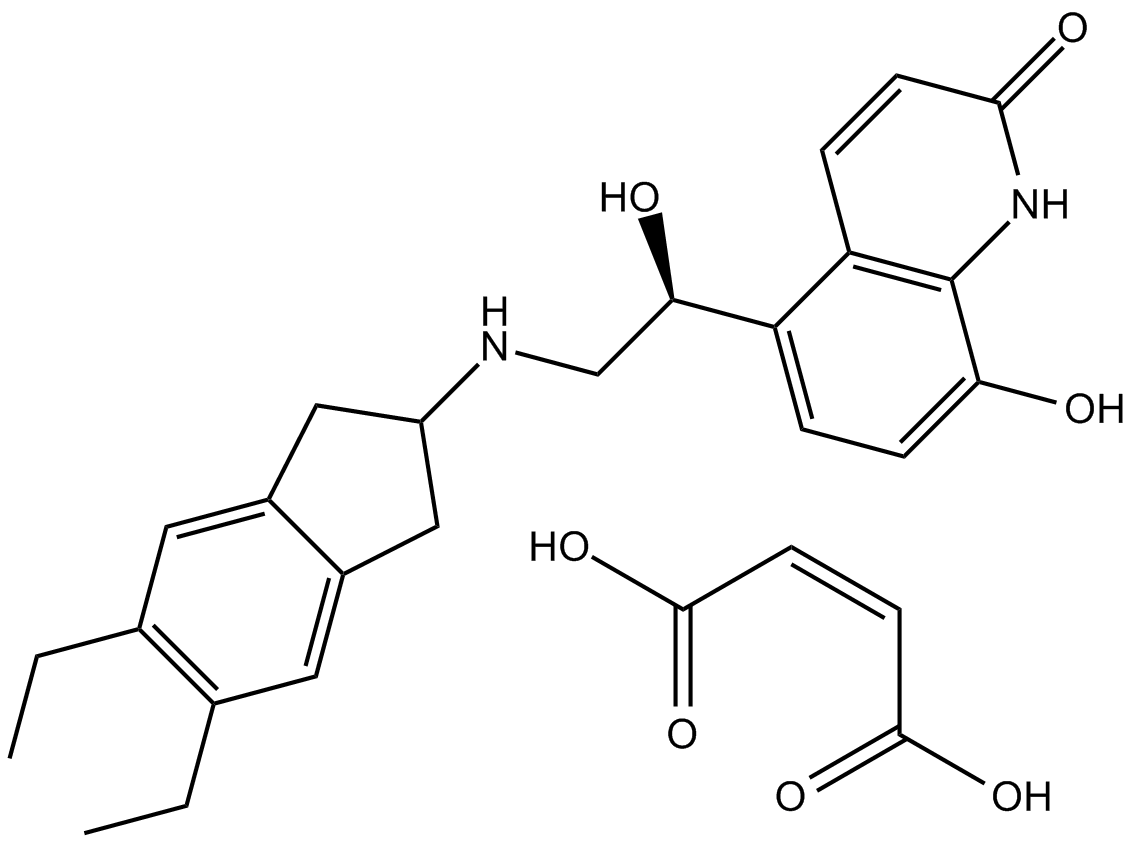
| Chemical Name | (Z)-but-2-enedioic acid;5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one | ||
| SMILES | CCC1=C(C=C2CC(CC2=C1)NCC(C3=C4C=CC(=O)NC4=C(C=C3)O)O)CC.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | IREJFXIHXRZFER-PCBAQXHCSA-N | ||
| Standard InChI | InChI=1S/C24H28N2O3.C4H4O4/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24;5-3(6)1-2-4(7)8/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29);1-2H,(H,5,6)(H,7,8)/b;2-1-/t22-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Indacaterol Maleate is an ultra-long-acting β-adrenoceptor agonist.
Target: β-adrenoceptor
Indacaterol inhibits cAMP production in Chinese hamster ovary cells stably transfected with human β2 adrenoceptors with pEC50 of 8.06. Indacaterol inhibits electrically induced contraction on the electrically stimulated guinea pig trachea in a concentration-dependent manner with pEC50 of 8.23. Indacaterol induces a concentration-dependent inotropic effect with maximal efficacy of 75% in the isolated guinea pig left atrium [1]. Indacaterol reverses the carbachol-induced contraction in a concentration-dependent manner with IC50 of 37 nM in human small airways. Indacaterol concentration dependently reverses the serotonin-induced contraction with IC50 of 10.5 nM in rat small airways. Indacaterol has the highest intrinsic efficacy of 53% in rat small airways and 73% in human small airways [2]. Indacaterol (6.7 μg/kg) inhibits 5-HT-induced bronchoconstriction with a maximal effect of 85% in the conscious guinea pig. Indacaterol (12.5 μg/kg) dose-dependently inhibits methacholine-induced bronchoconstriction with a maximal effect of 85% in the anesthetized rhesus monkey [1]. References: | |||||

Indacaterol Maleate Dilution Calculator

Indacaterol Maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9663 mL | 9.8317 mL | 19.6634 mL | 39.3267 mL | 49.1584 mL |
| 5 mM | 0.3933 mL | 1.9663 mL | 3.9327 mL | 7.8653 mL | 9.8317 mL |
| 10 mM | 0.1966 mL | 0.9832 mL | 1.9663 mL | 3.9327 mL | 4.9158 mL |
| 50 mM | 0.0393 mL | 0.1966 mL | 0.3933 mL | 0.7865 mL | 0.9832 mL |
| 100 mM | 0.0197 mL | 0.0983 mL | 0.1966 mL | 0.3933 mL | 0.4916 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Indacaterol is an ultra-long-acting β-adrenoceptor agonist with pKi of 7.36.
- Lovastatin
Catalog No.:BCN1060
CAS No.:75330-75-5
- H-Leucinol
Catalog No.:BCC2725
CAS No.:7533-40-6
- H-Pro-NH2
Catalog No.:BCC3018
CAS No.:7531-52-4
- Kukoamine A
Catalog No.:BCN3835
CAS No.:75288-96-9
- HEPES Sodium salt
Catalog No.:BCC7591
CAS No.:75277-39-3
- Nemonapride
Catalog No.:BCC7165
CAS No.:75272-39-8
- H-Trp-OMe.HCl
Catalog No.:BCC3114
CAS No.:7524-52-9
- H-Phe-OMe.HCl
Catalog No.:BCC3009
CAS No.:7524-50-7
- Ophiopogonanone A
Catalog No.:BCN6630
CAS No.:75239-63-3
- TAK-700 R-form
Catalog No.:BCC4203
CAS No.:752243-39-3
- 3,6-Bis(hydroxymethyl)durene
Catalog No.:BCC8598
CAS No.:7522-62-5
- Chikusetsusaponin IV
Catalog No.:BCN2683
CAS No.:7518-22-1
- Boc-Asn-OH
Catalog No.:BCC3071
CAS No.:7536-55-2
- Boc-Asp(OBzl)-OH
Catalog No.:BCC2608
CAS No.:7536-58-5
- EHT 1864
Catalog No.:BCC6075
CAS No.:754240-09-0
- Moxonidine
Catalog No.:BCC2142
CAS No.:75438-57-2
- CGP 37157
Catalog No.:BCC6943
CAS No.:75450-34-9
- N-Methylnuciferine
Catalog No.:BCN3971
CAS No.:754919-24-9
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- BI 2536
Catalog No.:BCC2081
CAS No.:755038-02-9
- BI6727 (Volasertib)
Catalog No.:BCC3886
CAS No.:755038-65-4
- Flupirtine maleate
Catalog No.:BCC4456
CAS No.:75507-68-5
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
Profile of inhaled glycopyrronium bromide as monotherapy and in fixed-dose combination with indacaterol maleate for the treatment of COPD.[Pubmed:25609944]
Int J Chron Obstruct Pulmon Dis. 2015 Jan 7;10:111-23.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality. The cornerstone of pharmacological treatment for COPD is bronchodilation. Inhaled glycopyrronium bromide is a long-acting muscarinic antagonist developed as a maintenance treatment for patients with COPD. Phase III trials have shown that glycopyrronium produces rapid and sustained bronchodilation with an efficacy similar to tiotropium and is well tolerated, with a low incidence of muscarinic side effects in patients with moderate to severe COPD. A combination of glycopyrronium bromide with Indacaterol Maleate (QVA149) has recently been approved as a once-daily maintenance therapy in adult patients with COPD. Phase III trials (the IGNITE program) with QVA149 have demonstrated significant improvements in lung function versus placebo, glycopyrronium, and tiotropium in patients with moderate to severe COPD, with no safety concerns of note. Hence QVA149 is a safe treatment option for moderate to severe COPD patients in whom long-acting muscarinic antagonist monotherapy is inadequate.
Spectrophotometric and spectrofluorimetric determination of indacaterol maleate in pure form and pharmaceutical preparations: application to content uniformity.[Pubmed:25620654]
Luminescence. 2015 Sep;30(6):891-7.
Two simple, rapid, sensitive and precise spectrophotometric and spectrofluorimetric methods were developed for the determination of Indacaterol Maleate in bulk powder and capsules. Both methods were based on the direct measurement of the drug in methanol. In the spectrophotometric method (Method I) the absorbance was measured at 259 nm. The absorbance-concentration plot was rectilinear over the range 1.0-10.0 microg mL(-1) with a lower detection limit (LOD) of 0.078 microg mL(-1) and lower quantification limit (LOQ) of 0.238 microg mL(-1). Meanwhile in the spectrofluorimetric method (Method II) the native fluorescence was measured at 358 nm after excitation at 258 nm. The fluorescence-concentration plot was rectilinear over the range of 1.0-40.0 ng mL(-1) with an LOD of 0.075 ng mL(-1) and an LOQ of 0.226 ng mL(-1). The proposed methods were successfully applied to the determination of Indacaterol Maleate in capsules with average percent recoveries +/- RSD% of 99.94 +/- 0.96 for Method I and 99.97 +/- 0.81 for Method II. In addition, the proposed methods were extended to a content uniformity test according to the United States Pharmacopoeia (USP) guidelines and were accurate, precise for the capsules studied with acceptance value 3.98 for Method I and 2.616 for Method II.
Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial.[Pubmed:25649209]
BMJ Open. 2015 Feb 3;5(2):e006131.
OBJECTIVE: To investigate the safety and efficacy of QMF149, a once-daily, fixed-dose combination of the long-acting beta2-agonist (LABA) Indacaterol Maleate and inhaled corticosteroid (ICS) mometasone furoate (MF) for the treatment of persistent asthma. The hypothesis was that QMF149 would not increase the risk of serious asthma exacerbations. SETTING: 174 research centres in nine countries. PARTICIPANTS: 1519 adolescents and adults with persistent asthma who were treated or qualified for treatment with combination LABA/ICS were randomised, and 1508 were included in the intention-to-treat analysis. INTERVENTION: Patients were randomised to QMF149 (Indacaterol Maleate 500 microg/MF 400 microg) or MF (400 microg) once daily via Twisthaler inhalation device in a double-blind, parallel-group study for 6-21 months. PRIMARY AND SECONDARY OUTCOME MEASURES: The primary end point was time to first serious asthma exacerbation (resulting in hospitalisation, intubation or death). The key secondary end point was annual rate of exacerbations requiring systemic corticosteroids. RESULTS: Treatment with QMF149 resulted in no significant difference in time to first serious exacerbation compared to MF (2 (0.3%) vs 6 events (0.8%); difference -0.52 percentage point; 95% CI -1.25 to 0.21, p=0.160, HR=0.31; 95% CI 0.06 to 1.54, p=0.151). QMF149 significantly reduced the annual rate of exacerbations requiring systemic corticosteroids (rate ratio=0.71; 95% CI 0.55 to 0.90, p=0.005). Proportions of patients experiencing adverse events were similar across groups (74.0% in the QMF149 group and 73.4% in the MF group). Serious adverse events occurred in 4% and 5.8% of patients in the QMF149 and MF groups, respectively. CONCLUSIONS: No significant difference was observed in the primary outcome of time to first serious asthma exacerbation in patients treated with QMF149 compared with patients treated with MF. Long-term treatment with QMF149 once daily had a favourable safety/efficacy profile in adolescent and adult patients with persistent asthma. TRIAL REGISTRATION NUMBER: ClinicalTrials.gov; NCT00941798.


