Incarvine CCAS# N/A |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | N/A | SDF | Download SDF |
| PubChem ID | 11222122 | Appearance | Powder |
| Formula | C21H29NO4 | M.Wt | 359.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
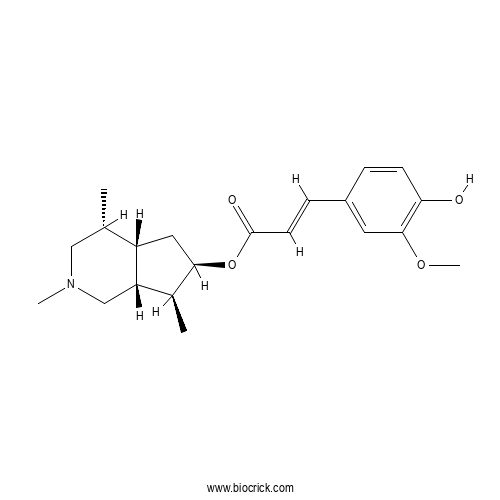
| Chemical Name | [(4R,4aS,6R,7S,7aR)-2,4,7-trimethyl-1,3,4,4a,5,6,7,7a-octahydrocyclopenta[c]pyridin-6-yl] (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | CC1CN(CC2C1CC(C2C)OC(=O)C=CC3=CC(=C(C=C3)O)OC)C | ||
| Standard InChIKey | OFDLBJAQGFWTKC-YJVHIDGBSA-N | ||
| Standard InChI | InChI=1S/C21H29NO4/c1-13-11-22(3)12-17-14(2)19(10-16(13)17)26-21(24)8-6-15-5-7-18(23)20(9-15)25-4/h5-9,13-14,16-17,19,23H,10-12H2,1-4H3/b8-6+/t13-,14-,16-,17-,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Incarvine C Dilution Calculator

Incarvine C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7816 mL | 13.9082 mL | 27.8164 mL | 55.6328 mL | 69.541 mL |
| 5 mM | 0.5563 mL | 2.7816 mL | 5.5633 mL | 11.1266 mL | 13.9082 mL |
| 10 mM | 0.2782 mL | 1.3908 mL | 2.7816 mL | 5.5633 mL | 6.9541 mL |
| 50 mM | 0.0556 mL | 0.2782 mL | 0.5563 mL | 1.1127 mL | 1.3908 mL |
| 100 mM | 0.0278 mL | 0.1391 mL | 0.2782 mL | 0.5563 mL | 0.6954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Incarvine A
Catalog No.:BCN0281
CAS No.:
- Vatalbinoside F
Catalog No.:BCN0280
CAS No.:
- Vatalbinoside I
Catalog No.:BCN0279
CAS No.:
- Vatalbinoside J
Catalog No.:BCN0278
CAS No.:
- Vatalbinoside H
Catalog No.:BCN0277
CAS No.:
- Vatalbinoside G
Catalog No.:BCN0276
CAS No.:
- Vatalbinoside C
Catalog No.:BCN0275
CAS No.:
- Vatalbinoside A
Catalog No.:BCN0274
CAS No.:
- Soyasaponin A1
Catalog No.:BCN0289
CAS No.:78693-94-4
- Soyasaponin A3
Catalog No.:BCN0288
CAS No.:114077-04-2
- Soyasaponin III
Catalog No.:BCN0287
CAS No.:55304-02-4
- Vaticanol B
Catalog No.:BCN0273
CAS No.:287101-83-1
- Incarvine D
Catalog No.:BCN0283
CAS No.:
- Incarvine E
Catalog No.:BCN0284
CAS No.:
- Incarvine F
Catalog No.:BCN0285
CAS No.:
- Incarvilone A
Catalog No.:BCN0286
CAS No.:
- Roridin A
Catalog No.:BCN0290
CAS No.:14729-29-4
- Agroclavine
Catalog No.:BCN0291
CAS No.:548-42-5
- Ergocornine
Catalog No.:BCN0292
CAS No.:564-36-3
- Procyanidin A4
Catalog No.:BCN0293
CAS No.:111466-29-6
- L-(±)-Alliin
Catalog No.:BCN0294
CAS No.:17795-26-5
- Andrograpanin
Catalog No.:BCN0295
CAS No.:82209-74-3
- Anemonin
Catalog No.:BCN0296
CAS No.:508-44-1
- Anisodine hydrobromide
Catalog No.:BCN0297
CAS No.:76822-34-9
Incarvine C suppresses proliferation and vasculogenic mimicry of hepatocellular carcinoma cells via targeting ROCK inhibition.[Pubmed:26510899]
BMC Cancer. 2015 Oct 28;15:814.
BACKGROUND: Studies have described vasculogenic mimicry (VM) as an alternative circulatory system to blood vessels in multiple malignant tumor types, including hepatocellular carcinoma (HCC). In the current study, we aimed to seek novel and more efficient treatment strategies by targeting VM and explore the underlying mechanisms in HCC cells. METHODS: Cell counting kit-8 (CCK-8) assay and colony survival assay were performed to explore the inhibitory effect of Incarvine C (IVC) on human cancer cell proliferation. Flow cytometry was performed to analyze the cell cycle distribution after DNA staining and cell apoptosis by the Annexin V-PE and 7-AAD assay. The effect of IVC on Rho-associated, coiled-coil-containing protein kinase (ROCK) was determined by western blotting and stress fiber formation assay. The inhibitory role of IVC on MHCC97H cell VM formation was determined by formation of tubular network structures on Matrigel in vitro, real time-qPCR, confocal microscopy and western blotting techniques. RESULTS: We explored an anti-metastatic HCC agent, IVC, derived from traditional Chinese medicinal herbs, and found that IVC dose-dependently inhibited the growth of MHCC97H cells. IVC induced MHCC97H cell cycle arrest at G1 transition, which was associated with cyclin-dependent kinase 2 (CDK-2)/cyclin-E1 degradation and p21/p53 up-regulation. In addition, IVC induced apoptotic death of MHCC97H cells. Furthermore, IVC strongly suppressed the phosphorylation of the ROCK substrate myosin phosphatase target subunit-1 (MYPT-1) and ROCK-mediated actin fiber formation. Finally, IVC inhibited cell-dominant tube formation in vitro, which was accompanied with the down-regulation of VM-key factors as detected by real time-qPCR and immunofluorescence. CONCLUSIONS: Taken together, the effective inhibitory effect of IVC on MHCC97H cell proliferation and neovascularization was associated with ROCK inhibition, suggesting that IVC may be a new potential drug candidate for the treatment of HCC.
Stereoselective synthesis of 7-epi-incarvilline.[Pubmed:23343424]
Org Lett. 2013 Feb 1;15(3):531-3.
The enantioselective synthesis of 7-epi-incarvilline for formal syntheses of (-)-incarvilline, (+)-Incarvine C, and (-)-incarvillateine is described. The key features of our synthesis involve (1) stereoselective construction of the optically active bicyclic lactone utilizing Pd(0)-catalyzed allylic alkylation, (2) efficient transformation of the bridged bicyclic lactone to the key bicyclic lactam skeleton, and (3) stereoselective elaborations of two stereocenters via a substrate-controlled catalytic hydrogenation and a 1,4-addition.
Total synthesis of (-)-incarvilline, (+)-incarvine C, and (-)-incarvillateine.[Pubmed:15600360]
J Am Chem Soc. 2004 Dec 22;126(50):16553-8.
The first total syntheses of new monoterpene alkaloids (-)-incarvilline, (+)-Incarvine C, and (-)-incarvillateine, corresponding to the natural enantiomers, have been accomplished. The strategy for the synthesis of these natural products utilized 6-epi-incarvilline as a common precursor, which was assembled by a three-component coupling reaction using (4S)-4-siloxy-2-cyclopenten-1-one to construct an appropriately trisubstituted cyclopentanone, followed by ring closure to the cis-perhydro-2-pyrindine skeleton by means of a reductive Heck-type reaction. Furthermore, topochemically controlled [2 + 2] photodimerization of cinnamic acid derivatives in the solid state for the stereospecific construction of a 1,2,3,4-tetrasubstituted cyclobutane ring was also investigated as a means to access (-)-incarvillateine.
Structure-antinociceptive activity studies of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis.[Pubmed:11301854]
Planta Med. 2001 Mar;67(2):114-7.
Incarvillateine (1), a new monoterpene alkaloid carrying a characteristic cyclobutane ring, has been found to show significant antinociceptive activity in a formalin-induced pain model in mice. To investigate the correlation between its structure and antinociceptive activity, and especially to study whether a cyclobutane ring is necessary or not for expression of activity, we evaluated the antinociceptive activity of two constructive units of incarvillateine, such as a monoterpene unit (incarvilline, 3) and a phenylpropanoid unit (ferulic acid, 2) in the formalin test, and compared activity of the units with that of incarvillateine. Furthermore, in order to obtain more information about the structure-activity relationships, monoterpene alkaloid derivatives, such as Incarvine C (5, a precursor of incarvillateine), incarvine A (4, an ester compound comprised of two monoterpene alkaloids and a monoterpene) and 3,3'-demethoxy-4,4'-dehydroxyincarvillateine (6, a synthetic new compound), were examined. The antinociceptive effect of 3,3'-demethoxy-4,4'-dehydroxyincarvillateine was equal to that of incarvillateine. Meanwhile, the other compounds exhibited no or weak activity. These results suggested that the cyclobutane moiety of incarvillateine plays an important role in expression of antinociceptive action.


