Impentamine dihydrobromideSelective H3 antagonist CAS# 149629-70-9 |
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Amantadine HCl
Catalog No.:BCC4465
CAS No.:665-66-7
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149629-70-9 | SDF | Download SDF |
| PubChem ID | 53439849 | Appearance | Powder |
| Formula | C8H17Br2N3 | M.Wt | 315.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VUF 4702 | ||
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
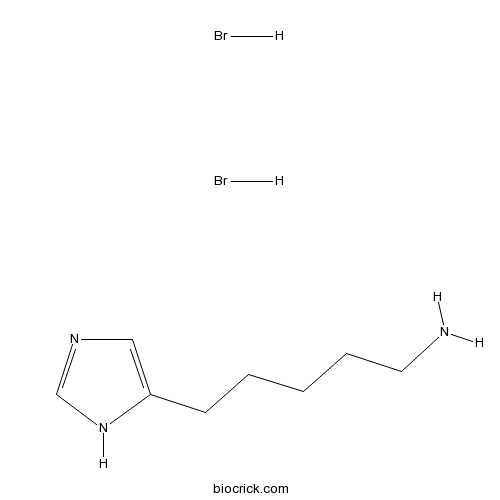
| Chemical Name | 5-(1H-imidazol-5-yl)pentan-1-amine;dihydrobromide | ||
| SMILES | C1=C(NC=N1)CCCCCN.Br.Br | ||
| Standard InChIKey | QSDXAAWBBPWGEL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H15N3.2BrH/c9-5-3-1-2-4-8-6-10-7-11-8;;/h6-7H,1-5,9H2,(H,10,11);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective histamine H3 receptor antagonist (pA2 = 8.4); displays > 30000-fold selectivity over H1 and H2 receptors. Can act as a partial agonist in SK-N-MC cells expressing human H3 receptors. Produces antinociception, possibly via a non-H1, -H2 or -H3 receptor-mediated mechanism, following central administration in vivo. |

Impentamine dihydrobromide Dilution Calculator

Impentamine dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1741 mL | 15.8705 mL | 31.741 mL | 63.482 mL | 79.3525 mL |
| 5 mM | 0.6348 mL | 3.1741 mL | 6.3482 mL | 12.6964 mL | 15.8705 mL |
| 10 mM | 0.3174 mL | 1.587 mL | 3.1741 mL | 6.3482 mL | 7.9352 mL |
| 50 mM | 0.0635 mL | 0.3174 mL | 0.6348 mL | 1.2696 mL | 1.587 mL |
| 100 mM | 0.0317 mL | 0.1587 mL | 0.3174 mL | 0.6348 mL | 0.7935 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stachybotramide
Catalog No.:BCN6969
CAS No.:149598-71-0
- Marmin
Catalog No.:BCN1665
CAS No.:14957-38-1
- Ganoderic acid AM1
Catalog No.:BCN2441
CAS No.:149507-55-1
- Hypocrellin C
Catalog No.:BCN3398
CAS No.:149457-83-0
- Traxillaside
Catalog No.:BCN6917
CAS No.:149415-62-3
- Poncirin
Catalog No.:BCN2590
CAS No.:14941-08-3
- NE 100 hydrochloride
Catalog No.:BCC7573
CAS No.:149409-57-4
- Cidofovir dihydrate
Catalog No.:BCC4247
CAS No.:149394-66-1
- UNC2881
Catalog No.:BCC5362
CAS No.:1493764-08-1
- 1,2,3,4,6-O-Pentagalloylglucose
Catalog No.:BCN2338
CAS No.:14937-32-7
- UNC2250
Catalog No.:BCC4876
CAS No.:1493694-70-4
- AACOCF3
Catalog No.:BCC7075
CAS No.:149301-79-1
- MOG (35-55)
Catalog No.:BCC3670
CAS No.:149635-73-4
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- Nafadotride
Catalog No.:BCC7025
CAS No.:149649-22-9
- Chrysosplenol D
Catalog No.:BCN1666
CAS No.:14965-20-9
- Isogambogic acid
Catalog No.:BCN3078
CAS No.:149655-52-7
- Isomorellinol
Catalog No.:BCN3075
CAS No.:149655-53-8
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Stachybotrolide
Catalog No.:BCN6968
CAS No.:149691-31-6
- AHU-377(Sacubitril)
Catalog No.:BCC4088
CAS No.:149709-62-6
- RS 23597-190 hydrochloride
Catalog No.:BCC6767
CAS No.:149719-06-2
- H-Arg-NH2.2HCl
Catalog No.:BCC2859
CAS No.:14975-30-5
- Tereticornate A
Catalog No.:BCN1667
CAS No.:149751-81-5
Constitutive activity of histamine h(3) receptors stably expressed in SK-N-MC cells: display of agonism and inverse agonism by H(3) antagonists.[Pubmed:11714875]
J Pharmacol Exp Ther. 2001 Dec;299(3):908-14.
Agonist-independent activity of G-protein-coupled receptor, also referred to as constitutive activity, is a well-documented phenomenon and has been reported recently for both the histamine H(1) and H(2) receptors. Using SK-N-MC cell lines stably expressing the human and rat H(3) receptors at physiological receptor densities (500-600 fmol/mg of protein), we show that both the rat and human H(3) receptors show a high degree of constitutive activity. The forskolin-mediated cAMP production in SK-N-MC cells is inhibited strongly upon expression of the G(i)-coupled H(3) receptor. The cAMP production can be further inhibited upon agonist stimulation of the H(3) receptor and can be enhanced by a variety of H(3) antagonists acting as inverse agonists at the H(3) receptor. Thioperamide, clobenpropit, and iodophenpropit raise the cAMP levels in SK-N-MC cells with potencies that match their receptor binding affinities. Surprisingly, impentamine and burimamide act as effective H(3) agonists. Modification of the amine group of impentamine dramatically affected the pharmacological activity of the ligand. Receptor affinity was reduced slightly for most impentamine analogs, but the functional activity of the ligands varied from agonist to neutral antagonist and inverse agonist, indicating that subtle changes in the chemical structures of impentamine analogs have major impact on the (de)activation steps of the H(3) receptor. In conclusion, upon stable expression of the rat and human H(3) receptor in SK-N-MC cells constitutive receptor activity is detected. In this experimental system, H(3) receptors ligands, previously identified as H(3) antagonists, cover the whole spectrum of pharmacological activities, ranging from full inverse agonists to agonists.
Antinociceptive activity of impentamine, a histamine congener, after CNS administration.[Pubmed:10072195]
Life Sci. 1999;64(5):PL79-86.
The brain neuromodulator histamine induces antinociception when administered directly into the rodent CNS. However, several compounds derived from H2 and H3 antagonists also produce antinociception after central administration. Pharmacological studies have shown that a prototype of these agents, improgan, induces analgesia that is not mediated by actions on known histamine receptors. Presently, the antinociceptive properties of a compound that chemically resembles both improgan and histamine were investigated in rats. Intraventricular (i.v.t.) administration of impentamine (4-imidazolylpentylamine) induced reversible, near-maximal antinociception on the hot plate and tail flick tests (15 microg, 98 nmol). The dose-response function was extremely steep, however, since other doses showed either no effect or behavioral toxicity. On the tail flick test, impentamine antinociception was resistant to antagonism by blockers of H1, H2, or H3 receptors, similar to characteristics previously found for improgan. In contrast, histamine antinociception was highly attenuated by H1 and H2 antagonists. These findings suggest that: 1) the histamine congener impentamine may induce antinociception by a mechanism similar to that produced by improgan, and 2) additional histamine receptors may be discovered that are linked to pain-attenuating processes.
Homologs of histamine as histamine H3 receptor antagonists: a new potent and selective H3 antagonist, 4(5)-(5-aminopentyl)-1H-imidazole.[Pubmed:7830269]
J Med Chem. 1995 Jan 20;38(2):266-71.
The influence of alkyl chain length variation on the histamine H3 receptor activity of histamine homologs 1 was investigated. A series of 4(5)-(omega-aminoalkyl)-1H-imidazoles 1 was prepared with an alkyl chain length varying from one methylene group to 10 methylene groups. Besides the H3 activity, the affinities of these compounds for the H1 and H2 receptors were determined. The ethylene chain of histamine is optimal for agonistic activity on all three histamine receptor subtypes. For the H3 receptor, elongation of the alkyl chain from three methylene groups on leads to compounds with antagonistic properties. 4(5)-(5-Aminopentyl)-1H-imidazole (impentamine, 1e) is the most potent and selective H3 antagonist from this series of 4(5)-(omega-aminoalkyl)-1H-imidazoles 1, with a pA2 value of 8.4 (on guinea pig jejunum). A specific antagonistic binding site for this compound is proposed.


