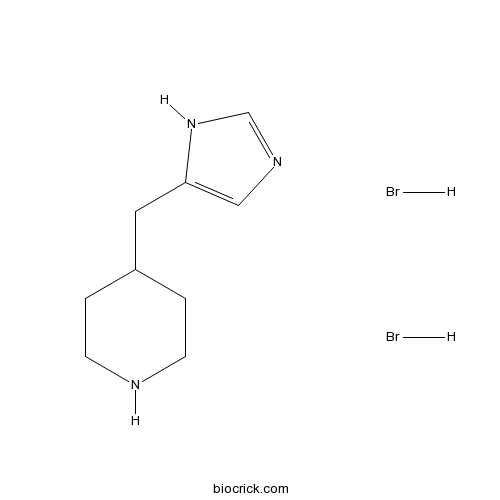
Immepip dihydrobromideH4 and H3 agonist CAS# 164391-47-3 |
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Amantadine HCl
Catalog No.:BCC4465
CAS No.:665-66-7
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 164391-47-3 | SDF | Download SDF |
| PubChem ID | 22315065 | Appearance | Powder |
| Formula | C9H17Br2N3 | M.Wt | 327.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 4-(1H-imidazol-5-ylmethyl)piperidine;dihydrobromide | ||
| SMILES | C1CNCCC1CC2=CN=CN2.Br.Br | ||
| Standard InChIKey | YGNJPNRDGXJQJX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H15N3.2BrH/c1-3-10-4-2-8(1)5-9-6-11-7-12-9;;/h6-8,10H,1-5H2,(H,11,12);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent histamine H3 receptor agonist. Also binds to H4 receptors (Ki values are 0.4 and 9 nM at human recombinant H3 and H4 receptors respectively). Equipotent to or slightly more active than (R)-α-methylhistamine at H3 receptors. |

Immepip dihydrobromide Dilution Calculator

Immepip dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0575 mL | 15.2877 mL | 30.5754 mL | 61.1509 mL | 76.4386 mL |
| 5 mM | 0.6115 mL | 3.0575 mL | 6.1151 mL | 12.2302 mL | 15.2877 mL |
| 10 mM | 0.3058 mL | 1.5288 mL | 3.0575 mL | 6.1151 mL | 7.6439 mL |
| 50 mM | 0.0612 mL | 0.3058 mL | 0.6115 mL | 1.223 mL | 1.5288 mL |
| 100 mM | 0.0306 mL | 0.1529 mL | 0.3058 mL | 0.6115 mL | 0.7644 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-D-Phe(4-NH2)-OH
Catalog No.:BCC3155
CAS No.:164332-89-2
- Phosphoramidon Disodium Salt
Catalog No.:BCC5484
CAS No.:164204-38-0
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
- LY 311727
Catalog No.:BCC7728
CAS No.:164083-84-5
- Clerodenoside A
Catalog No.:BCN1725
CAS No.:164022-75-7
- Longiferone B
Catalog No.:BCN7378
CAS No.:1639810-67-5
- Kaempferol-3-O-(6''-O-cis-coumaryl)glucoside
Catalog No.:BCN1538
CAS No.:163956-16-9
- 12E,14-Labdadien-20,8beta-olide
Catalog No.:BCN7395
CAS No.:1639257-37-6
- 8(17),12E,14-Labdatrien-20-oic acid
Catalog No.:BCN7396
CAS No.:1639257-36-5
- Androstenediol-3-acetate
Catalog No.:BCC8829
CAS No.:1639-43-6
- Salvianolic acid Y
Catalog No.:BCN8123
CAS No.:1638738-76-7
- 7beta-Hydroxyrutaecarpine
Catalog No.:BCN6500
CAS No.:163815-35-8
- KPT-9274
Catalog No.:BCC6461
CAS No.:1643913-93-2
- Aldicarb sulfone
Catalog No.:BCC5476
CAS No.:1646-88-4
- Efinaconazole
Catalog No.:BCC5523
CAS No.:164650-44-6
- Dutasteride
Catalog No.:BCC1097
CAS No.:164656-23-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- 4-(3,4-Dimethoxyphenyl)-3-butene-1,2-diol
Catalog No.:BCN1537
CAS No.:164661-12-5
- Podophyllotoxin 4-O-glucoside
Catalog No.:BCN8064
CAS No.:16481-54-2
- G-5555
Catalog No.:BCC8095
CAS No.:1648863-90-4
- Azaperone
Catalog No.:BCC4630
CAS No.:1649-18-9
- ML352
Catalog No.:BCC6434
CAS No.:1649450-12-3
- N9-Methylharman
Catalog No.:BCN3368
CAS No.:16498-64-9
- Calyxin B
Catalog No.:BCN1726
CAS No.:164991-53-1
TRPV1 and PLC Participate in Histamine H4 Receptor-Induced Itch.[Pubmed:26819760]
Neural Plast. 2016;2016:1682972.
Histamine H4 receptor has been confirmed to play a role in evoking peripheral pruritus. However, the ionic and intracellular signaling mechanism of activation of H4 receptor on the dorsal root ganglion (DRG) neurons is still unknown. By using cell culture and calcium imaging, we studied the underlying mechanism of activation of H4 receptor on the DRG neuron. Immepip dihydrobromide (immepip)-a histamine H4 receptor special agonist under cutaneous injection-obviously induced itch behavior of mice. Immepip-induced scratching behavior could be blocked by TRPV1 antagonist AMG9810 and PLC pathway inhibitor U73122. Application of immepip (8.3-50 muM) could also induce a dose-dependent increase in intracellular Ca(2+) ([Ca(2+)]i) of DRG neurons. We found that 77.8% of the immepip-sensitized DRG neurons respond to the TRPV1 selective agonist capsaicin. U73122 could inhibit immepip-induced Ca(2+) responses. In addition, immepip-induced [Ca(2+)]i increase could be blocked by ruthenium red, capsazepine, and AMG9810; however it could not be blocked by TRPA1 antagonist HC-030031. These results indicate that TRPV1 but not TRPA1 is the important ion channel to induce the DRG neurons' responses in the downstream signaling pathway of histamine H4 receptor and suggest that TRPV1 may be involved in the mechanism of histamine-induced itch response by H4 receptor activation.
Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow.[Pubmed:11179434]
Mol Pharmacol. 2001 Mar;59(3):420-6.
Histamine is a multifunctional hormone that regulates smooth muscle contraction in the airways, acid secretion in the gut, and neurotransmitter release in the central nervous system through three well characterized receptor subtypes, H(1), H(2), H(3), respectively. As part of a directed effort to discover novel G-protein-coupled receptors through homology searching of genomic databases, we identified a partial clone (GPCR105) that had significant homology to the recently identified histamine H(3) receptor cDNA. Expression of the full-length human GPCR105 in cells confers the ability to bind [(3)H]histamine with high affinity (K(D) = 5 nM). GPCR105 is pharmacologically similar to the histamine H(3) receptor in that it binds many of the known H(3) agonists and antagonists, albeit with a different rank order of affinity/potency. GPCR105 does not bind (i.e., K(D) > 10 microM) all tested H(1) and H(2) receptor antagonists such as diphenhydramine, loratadine, ranitidine, and cimetidine, but has modest affinity for the H(2) receptor agonist, dimaprit (377 nM). Whereas the H(3) receptor is expressed almost exclusively in nervous tissues, GPRC105 is expressed primarily in bone marrow and eosinophils. Together, these data demonstrate that GPCR105 is a novel histamine receptor structurally and pharmacologically related to the H(3) receptor. However, its unique expression profile and physiological role suggest that GPCR105 is a fourth histamine receptor subtype (H(4)) and may be a therapeutic target for the regulation of immune function, particularly with respect to allergy and asthma.
Cardiovascular effects of selective agonists and antagonists of histamine H3 receptors in the anaesthetized rat.[Pubmed:7675114]
Naunyn Schmiedebergs Arch Pharmacol. 1995 Jun;351(6):569-75.
The cardiovascular responses to a series of selective histamine H3 receptor agonists, (R) alpha-methylhistamine, imetit and immepip and selective antagonists, thioperamide, clobenpropit and clophenpropit, were studied in anaesthetized rats. At 0.003-1 mumol/kg i.v. doses, H3 agonists failed to produce any significant change in the basal blood pressure and heart rate. Larger doses of (R) alpha-methylhistamine increased the blood pressure and heart rate and higher doses of imetit caused vasodepressor responses and reduced heart rate, whereas immepip proved virtually inactive. While (R) alpha-methylhistamine-induced effects were not blocked by histamine H1-, H2- and H3-receptor antagonists, they were however reduced by idazoxan and propranolol, which indicates that the mechanisms involved are adrenergic. The effects induced by imetit are not related to histamine H3 receptors but are mediated by indirect (via 5HT3 receptors) cholinergic mechanisms, since these effects were prevented by 1 mg/kg i.v. atropine and by 0.1 mg/kg i.v. ondansetron. Similarly, the H3 antagonists per se failed to change basal cardiovascular function up to 10 mumol/kg i.v. and only at 30 mumol/kg i.v. were marked decreases observed in the blood pressure and heart rate with a significant reduction in the effects of noradrenaline. These data indicate that in anaesthetized rats, histamine H3 receptor activation or blockade has no effect on basal cardiovascular function. The effects recorded after the administration of large doses of (R) alpha-methylhistamine and imetit are clearly unrelated to histamine H3 receptors and should be taken into account when using these compounds as H3 ligands for "in vivo" experiments.


