IchanginCAS# 10171-61-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10171-61-6 | SDF | Download SDF |
| PubChem ID | 441801 | Appearance | Powder |
| Formula | C26H32O9 | M.Wt | 488.5 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
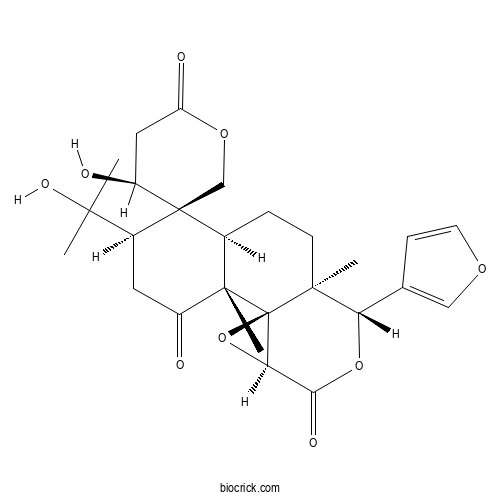
| Chemical Name | (1R,2R,4'S,5R,6R,7R,10S,11S,14S)-11-(furan-3-yl)-4'-hydroxy-5-(2-hydroxypropan-2-yl)-2,10-dimethylspiro[12,15-dioxatetracyclo[8.5.0.01,14.02,7]pentadecane-6,5'-oxane]-2',3,13-trione | ||
| SMILES | CC12CCC3C(C14C(O4)C(=O)OC2C5=COC=C5)(C(=O)CC(C36COC(=O)CC6O)C(C)(C)O)C | ||
| Standard InChIKey | GNNAZOFNKOMONV-MSGMIQHVSA-N | ||
| Standard InChI | InChI=1S/C26H32O9/c1-22(2,31)15-9-16(27)24(4)14(25(15)12-33-18(29)10-17(25)28)5-7-23(3)19(13-6-8-32-11-13)34-21(30)20-26(23,24)35-20/h6,8,11,14-15,17,19-20,28,31H,5,7,9-10,12H2,1-4H3/t14-,15-,17-,19-,20+,23-,24-,25+,26+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ichangin Dilution Calculator

Ichangin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0471 mL | 10.2354 mL | 20.4708 mL | 40.9417 mL | 51.1771 mL |
| 5 mM | 0.4094 mL | 2.0471 mL | 4.0942 mL | 8.1883 mL | 10.2354 mL |
| 10 mM | 0.2047 mL | 1.0235 mL | 2.0471 mL | 4.0942 mL | 5.1177 mL |
| 50 mM | 0.0409 mL | 0.2047 mL | 0.4094 mL | 0.8188 mL | 1.0235 mL |
| 100 mM | 0.0205 mL | 0.1024 mL | 0.2047 mL | 0.4094 mL | 0.5118 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Obacunone 17-O-glucoside
Catalog No.:BCX0428
CAS No.:123564-64-7
- 3-epi-Actinidic acid
Catalog No.:BCX0427
CAS No.:143839-01-4
- (±)-Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCX0426
CAS No.:85165-02-2
- (-)-Isolariciresinol
Catalog No.:BCX0425
CAS No.:110268-37-6
- 21,23-Dihydro-21-hydroxy-23-oxonomilin
Catalog No.:BCX0424
CAS No.:2243600-32-8
- Procymidone
Catalog No.:BCX0423
CAS No.:32809-16-8
- Azoxystrobin
Catalog No.:BCX0422
CAS No.:131860-33-8
- 5α,6α-Epoxyergosta-8,22-diene-3β,7α-diol
Catalog No.:BCX0421
CAS No.:16250-61-6
- 20(R)-Hydroxypregn-4-en-3-one
Catalog No.:BCX0420
CAS No.:145-15-3
- 5α,6α-Epoxyergosta-8(14),22-diene-3β,7α-diol
Catalog No.:BCX0419
CAS No.:22259-18-3
- Lycoperodine 1
Catalog No.:BCX0418
CAS No.:6052-68-2
- Nomilin 17-O-glucoside
Catalog No.:BCX0417
CAS No.:123564-62-5
- Deacetylnomilinic acid
Catalog No.:BCX0430
CAS No.:35930-21-3
- Anisyl alcohol
Catalog No.:BCX0431
CAS No.:105-13-5
- Carbendazim
Catalog No.:BCX0432
CAS No.:10605-21-7
- (2Z,6E)-Farnesyl acetate
Catalog No.:BCX0433
CAS No.:40266-29-3
- Isolimonic acid
Catalog No.:BCX0434
CAS No.:74729-97-8
- Anibadimer A
Catalog No.:BCX0435
CAS No.:23768-65-2
- 5-Deoxyisorhoifolin
Catalog No.:BCX0436
CAS No.:2055239-29-5
- 1(10)Z,4Z-Furanodienone
Catalog No.:BCX0437
CAS No.:88010-63-3
- Thalifaronine
Catalog No.:BCX0438
CAS No.:105458-70-6
- Huazhongilexol
Catalog No.:BCX0439
CAS No.:161407-80-3
- Thalifaramine
Catalog No.:BCX0440
CAS No.:105437-16-9
- (12Z)-Labda-8(17),12-diene-14,15,16-triol
Catalog No.:BCX0441
CAS No.:1630864-26-4
Virtual screening of phytochemicals by targeting multiple proteins of severe acute respiratory syndrome coronavirus 2: Molecular docking and molecular dynamics simulation studies.[Pubmed:36442514]
Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221142793.
OBJECTIVE: Medicinal herbs are being investigated for medicationhg development against SARS-CoV-2 as a rich source of bioactive chemicals. One of the finest approaches for finding therapeutically effective drug molecules in real time is virtual screening scheme such as molecular docking in conjunction with molecular dynamics (MD) simulation. These virtual techniques provide an ample opportunity for the screening of plausible inhibitors of SARS-CoV-2 different target proteins from a comprehensive and extensive phytochemical library. The study was designed to identify potential phytochemicals by virtual screening against different receptor proteins. METHODS: In the current study, a library of plant secondary metabolites was created by manually curating 120 phytochemicals known to have antimicrobial as well as antiviral properties. In the current study, different potential phytochemicals were identified by virtual screening against various selected receptor proteins (i.e., viral main proteases, RNA-dependent RNA polymerase (RdRp), ADP ribose phosphatase, nonstructural proteins NSP7, NSP8, and NSP9) which are key proteins responsible for transcription, replication and maturation of SARS-CoV-2 in the host. Top three phytochemicals were selected against each viral receptor protein based on their best S-scores, RMSD values, molecular interactions, binding patterns and drug-likeness properties. RESULTS: The results of molecular docking study revealed that phytochemicals (i.e., baicalin, betaxanthin, epigallocatechin, fomecin A, gallic acid, hortensin, Ichangin, kaempferol, limonoic acid, myricetin hexaacetat, pedalitin, quercetin, quercitrin, and silvestrol) have strong antiviral potential against SARS-CoV-2. Additionally, the reported preeminent reliable phytochemicals also revealed toxicity by no means during the evaluation through ADMET profiling. Moreover, the MD simulation study also exhibited thermal stability and stable binding affinity of the pedalitin with SARS-CoV-2 RdRp and SARS-CoV-2 main protease which suggests appreciable efficacy of the lead optimization. CONCLUSION: The biological activity and pharmacologically distinguishing characteristics of these lead compounds also satisfied as repurposing antiviral drug contenders and are worth substantial evaluation in the biological laboratory for the recommendation of being plausible antiviral drug candidates against SARS-CoV-2.
Interaction of selected terpenoids with two SARS-CoV-2 key therapeutic targets: An in silico study through molecular docking and dynamics simulations.[Pubmed:34116362]
Comput Biol Med. 2021 Jul;134:104538.
The outbreak of COVID-19 disease caused by SARS-CoV-2, along with the lack of targeted medicaments, forced the scientific world to search for new antiviral formulations. In the current emergent situation, drug repurposing of well-known traditional and/or approved drugs could be the most effective strategy. Herein, through computational approaches, we aimed to screen 14 natural compounds from limonoids and terpenoids class for their ability to inhibit the key therapeutic target proteins of SARS-CoV-2. Among these, some limonoids, namely deacetylnomilin, Ichangin and nomilin, and the terpenoid beta-amyrin provided good interaction energies with SARS-CoV-2 3CL hydrolase (Mpro) in molecular dynamic simulation. Interestingly, deacetylnomilin and Ichangin showed direct interaction with the catalytic dyad of the enzyme so supporting their potential role in preventing SARS-CoV-2 replication and growth. On the contrary, despite the good affinity with the spike protein RBD site, all the selected phytochemicals lose contact with the amino acid residues over the course of 120ns-long molecular dynamics simulations therefore suggesting they scarcely can interfere in SARS-CoV-2 binding to the ACE2 receptor. The in silico analyses of docking score and binding energies, along with predicted pharmacokinetic profiles, indicate that these triterpenoids might have potential as inhibitors of SARS-CoV-2 Mpro, recommending further in vitro and in vivo investigations for a complete understanding and confirmation of their inhibitory potential.
Isolimonic acid interferes with Escherichia coli O157:H7 biofilm and TTSS in QseBC and QseA dependent fashion.[Pubmed:23153211]
BMC Microbiol. 2012 Nov 15;12:261.
BACKGROUND: E. coli O157:H7 (EHEC) is an important human pathogen. The antibiotic treatment of EHEC reportedly results in release of Shiga toxin and is therefore discouraged. Consequently, alternative preventive or therapeutic strategies for EHEC are required. The objective of the current study was to investigate the effect of citrus limonoids on cell-cell signaling, biofilm formation and type III secretion system in EHEC. RESULTS: Isolimonic acid and Ichangin were the most potent inhibitors of EHEC biofilm (IC25=19.7 and 28.3 muM, respectively) and adhesion to Caco-2 cells. The qPCR analysis revealed that isolimonic acid and Ichangin repressed LEE encoded genes by approximately 3 to 12 fold. In addition, flhDC was repressed by the two limonoids ( approximately 3 to 7 fold). Further studies suggested that isolimonic acid interferes with AI-3/epinephrine activated cell-cell signaling pathway. Loss of biofilm inhibitory activity of isolimonic acid in DeltaqseBC mutant, which could be restored upon complementation, suggested a dependence on functional QseBC. Additionally, overexpression of qseBC in wild type EHEC abated the inhibitory effect of isolimonic acid. Furthermore, the isolimonic acid failed to differentially regulate ler in DeltaqseA mutant, while plasmid borne expression of qseA in DeltaqseA background restored the repressive effect of isolimonic acid. CONCLUSIONS: Altogether, results of study seem to suggest that isolimonic acid and Ichangin are potent inhibitors of EHEC biofilm and TTSS. Furthermore, isolimonic acid appears to interfere with AI-3/epinephrine pathway in QseBC and QseA dependent fashion.
Secondary metabolites of ponderosa lemon (Citrus pyriformis) and their antioxidant, anti-inflammatory, and cytotoxic activities.[Pubmed:21950163]
Z Naturforsch C J Biosci. 2011 Jul-Aug;66(7-8):385-93.
Column chromatography of the dichloromethane fraction from an aqueous methanolic extract of fruit peel of Citrus pyriformis Hassk. (Rutaceae) resulted in the isolation of seven compounds including one coumarin (citropten), two limonoids (limonin and deacetylnomilin), and four sterols (stigmasterol, ergosterol, sitosteryl-3-beta-D-glucoside, and sitosteryl-6'-O-acyl-3-beta-D-glucoside). From the ethyl acetate fraction naringin, hesperidin, and neohesperidin were isolated. The dichloromethane extract of the defatted seeds contained three additional compounds, nomilin, Ichangin, and cholesterol. The isolated compounds were identified by MS (EI, CI, and ESI), 1H, 13C, and 2D-NMR spectral data. The limonoids were determined qualitatively by LC-ESI/MS resulting in the identification of 11 limonoid aglycones. The total methanolic extract of the peel and the petroleum ether, dichloromethane, and ethyl acetate fractions were screened for their antioxidant and anti-inflammatory activities. The ethyl acetate fraction exhibited a significant scavenging activity for DPPH free radicals (IC50 = 132.3 microg/mL). The petroleum ether fraction inhibited 5-lipoxygenase with IC50 = 30.6 microg/mL indicating potential anti-inflammatory properties. Limonin has a potent cytotoxic effect against COS7 cells [IC50 = (35.0 +/- 6.1) microM] compared with acteoside as a positive control [IC50 = (144.5 +/- 10.96) microM].
seco-limonoids and quinoline alkaloids from Raputia heptaphylla and their antileishmanial activity.[Pubmed:21720036]
Chem Pharm Bull (Tokyo). 2011;59(7):855-9.
A novel seco-limonoid, rel-(1S,5R,9S,7R,8S,9R,10S,11R,13S,14R,15R,17R)-11,19-dihydroxy-7-acetoxy-7-deoxoIchangin (raputiolide) (1), and two novel quinolone alkaloids N-methyl-2-phenoxyquinolin-4(1H)-one (heptaphyllone A) (2) and 6-methylbenzofuro[2,3-b]quinolin-4(1H)-one (heptaphyllone B) (3), along with the known seco-limonoid Ichangin (4), were isolated from Raputia heptaphylla PITTIER (Rutaceae) stem bark. Five known alkaloids, N-methyl-8-methoxyflindersine (5), skimmianine (6), kokusaginine (7), dictamnine (8) and flindersiamine (9), were also isolated from R. heptaphylla leaves. Their structures were established on the basis of full spectroscopic data interpretation supported by data from the pertinent literature. seco-Limonoid 1 configuration was determined by enhanced nuclear Overhauser effect spectroscopy (NOESY) experiments and density functional theory (DFT) molecular modeling. The antileishmanial effect of the isolated compounds was evaluated on Leishmania Viannia panamensis (promastigotes and amastigotes). Whereas alkaloids 2-3, 6-8 and limonoid 4 exhibited no significant parasitocide activity against internalized L. (V.) panamensis amastigotes, limonoid 1 and alkaloid 5 had leishmanicidal activity on intracellular amastigotes (EC(5)(0): 8.7 microg/ml) and promastigotes (EC(50): 14.3 microg/ml), respectively.
Simultaneous separation and identification of limonoids from citrus using liquid chromatography-collision-induced dissociation mass spectra.[Pubmed:21171170]
J Sep Sci. 2011 Jan;34(1):2-10.
Limonoids are considered as potential cancer chemopreventive agents and are widely distributed in the Citrus genus as aglycones and glucosides. In the present study, reversed-phase HPLC coupled with CID mass spectra was developed for the simultaneous separation and identification of aglycones and glucosides of limonoids from citrus. Five aglycones such as limonin, deacetyl nomilin, Ichangin, isolimonoic acid and nomilin were identified by positive ion CID MS/MS, whereas five glucosides, viz. limonin glucoside, isoobacunoic acid glucoside, obacunone glucoside, deacetyl nomilinic acid glucoside and nomilinic acid glucoside were analyzed by negative ion CID mass spectra. The developed method was successfully applied to complex citrus samples for the separation and identification of aglycones and glucosides. Citrus seeds were extracted with methanol and partially purified and analyzed by LC-CID mass spectra. The separation was achieved by C-18 column; eight limonoids were identified by comparing the retention times and mass spectral fragmentation. To the best of our knowledge, this is the first report on the identification of citrus limonoids using CID technique.
Citrus limonoids interfere with Vibrio harveyi cell-cell signalling and biofilm formation by modulating the response regulator LuxO.[Pubmed:20864476]
Microbiology (Reading). 2011 Jan;157(Pt 1):99-110.
Citrus limonoids are unique secondary metabolites, characterized by a triterpenoid skeleton with a furan ring. Studies have demonstrated beneficial health properties of limonoids. In addition, certain citrus limonoids play a role in plant defence against insect pests. In the present study, five limonoids were purified from sour orange and evaluated for their ability to inhibit cell-cell signalling. The purified limonoids were tested for their ability to interfere with cell-cell signalling and biofilm formation in Vibrio harveyi. Isolimonic acid, deacetylnomilinic acid glucoside and Ichangin demonstrated significant inhibition of autoinducer-mediated cell-cell signalling and biofilm formation. Furthermore, isolimonic acid and Ichangin treatment resulted in induced expression of the response regulator gene luxO. In addition, luxR promoter activity was not affected by isolimonic acid or Ichangin. Therefore, the ability of isolimonic acid and Ichangin to interfere with cell-cell signalling and biofilm formation seems to stem from the modulation of luxO expression. The results suggest that isolimonic acid and Ichangin are potent modulators of bacterial cell-cell signalling.
Simultaneous determination of citrus limonoid aglycones and glucosides by high performance liquid chromatography.[Pubmed:17448343]
Anal Chim Acta. 2007 May 8;590(2):180-6.
High performance liquid chromatography (HPLC) method has been developed for simultaneous quantification of limonoid aglycones and glucosides on a reversed phase C18 column using a binary solvent system, coupled with diode array detector. Seven limonoids such as limonin, nomilin, isolimonic acid, Ichangin, isoobacunoic acid, limonin 17-beta-D glucopyranoside and deacetyl nomilinic acid 17-beta-D glucopyranoside were separated and detected at 210 nm. Furthermore, limonoids were separated, identified and quantified in four varieties of citrus fruits and seeds using developed method. Limonin and limonin glucoside were found to be the predominant limonoid aglycone and glucoside, respectively, in all tested samples. The sensitivity of the method was found to be 0.25-0.50 microg for tested limonoids.
Limonoids from Citrus reticulata.[Pubmed:12710721]
Z Naturforsch C J Biosci. 2003 Mar-Apr;58(3-4):165-70.
The seeds of Citrus reticulata afforded the new limonoid derivative, isolimonexic acid methyl ether, in addition to the previously isolated limonin, deacetylnomilin, obacunone and Ichangin. The structure elucidation was achieved primarily through 1D and 2-D-NMR analyses. The marginal antimalarial activity of isolimonexic acid methyl ether is reported.


