Epacadostatpotent and selective inhibitor of IDO1 CAS# 1204669-58-8 |
- NFAT Inhibitor
Catalog No.:BCC2463
CAS No.:249537-73-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1204669-58-8 | SDF | Download SDF |
| PubChem ID | 44608567 | Appearance | Powder |
| Formula | C11H13BrFN7O4S | M.Wt | 438.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | INCB-024360; IDO Inhibitor 1; 1204669-37-3 | ||
| Solubility | DMSO : 100 mg/mL (228.19 mM; Need ultrasonic) | ||
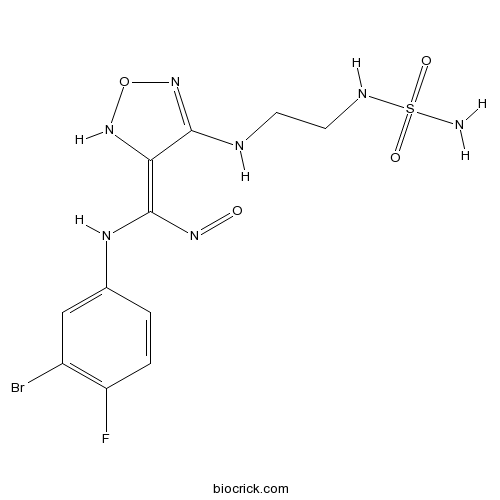
| Chemical Name | (3E)-3-[(3-bromo-4-fluoroanilino)-nitrosomethylidene]-4-[2-(sulfamoylamino)ethylamino]-1,2,5-oxadiazole | ||
| SMILES | C1=CC(=C(C=C1NC(=C2C(=NON2)NCCNS(=O)(=O)N)N=O)Br)F | ||
| Standard InChIKey | YPBKTZBXSBLTDK-PKNBQFBNSA-N | ||
| Standard InChI | InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16-17,19H,3-4H2,(H,15,20)(H2,14,22,23)/b11-9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | INCB024360 is a potent inhibitor of IDO1 with an IC50 value of 10 nM. | |||||
| Targets | IDO1 | |||||
| IC50 | 10 nM | |||||

Epacadostat Dilution Calculator

Epacadostat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2819 mL | 11.4095 mL | 22.8191 mL | 45.6381 mL | 57.0477 mL |
| 5 mM | 0.4564 mL | 2.2819 mL | 4.5638 mL | 9.1276 mL | 11.4095 mL |
| 10 mM | 0.2282 mL | 1.141 mL | 2.2819 mL | 4.5638 mL | 5.7048 mL |
| 50 mM | 0.0456 mL | 0.2282 mL | 0.4564 mL | 0.9128 mL | 1.141 mL |
| 100 mM | 0.0228 mL | 0.1141 mL | 0.2282 mL | 0.4564 mL | 0.5705 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
INCB024360 is a potent and selective inhibitor of IDO1 with IC50 value of 10 nM. [1]
IDO means indoleamine-pyrrole 2, 3-dioxygenase. IDO is an enzyme which is encoded by the IDO1 gene. IDO is the rate-limiting and first enzyme of tryptophan which is one amino acid of human catabolism through kynurenine pathway. The decrease of L-tryptophan can cause halted growth of T cells as well as microbes. IDO belongs to immunomodulatory enzyme. It is produced by some activated macrophages and immunoregulatory cells. IDO is overexpressed in a wide range of cancer cells such as lung, prostatic, pancreatic, colorectal cancer. It is indentified to help cancer cells to escape the immune system by reducing the level of L-tryptophan in the microenvironment of cells.[2]
In Hela cells, INCB024360 selectively inhibits the activity of human IDO1 with IC50 values of about 10nM. On the other hand INCB024360demonstrates little inhibition activity against human IDO1 or TDO (tryptophan 2, 3-dioxygenase). In coculture systems of human dendritic cells with allogeneic lymphocytes, INCB024360 inhibit T-cell proliferation and cytokine production and influence the viability of NK cells. INCB024360 also increase CD86 expression and promote activation T cells by DCs.
In mice bearing IDO1-expressing PAN02 pancreatic tumour, ICB024360 significantly inhibit tumour growth in lymphocyte-dependent manner.[1] INCB024360 decreased plasma kynurenine levels by inhibiting the activity of IDO1 at 50 mg/kg in native C57BL/6 mice. INCB024360 also inhibit tumor growth at 100mg/kg in CT26 tumor bearing mice.[3]
References:
[1]. Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M et al: Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood, 115(17):3520-3530.
[2]. Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ: Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003, 9(10):1269-1274.
[3]. Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW et al: Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther, 9(2):489-498.
IDO inhibitor 1 is a potent and novel indoleamine-2,3 dioxygenase (IDO) inhibitor with IC50 value <100 nM.
IDO is an enzyme that catalyzes the degradation of the essential amino acid L-tryptophan to N-formylkynurenine and permits tumor cells to escape the immune system.
Reference:
[1]. Andrew P. Combs, et al. 1,2,5-oxadiazoles as inhibitors of indoleamine 2,3-dioxygenase. Patent Numeber: EP 2315756 A2
http://www. google.com/patents/EP2315756A2?cl=en
- Pinanediol talabostat boronate
Catalog No.:BCC1640
CAS No.:1204669-37-3
- Ganoderenic acid H
Catalog No.:BCN2447
CAS No.:120462-48-8
- Ganoderenic acid F
Catalog No.:BCN2446
CAS No.:120462-47-7
- Lophanthoidin F
Catalog No.:BCN6093
CAS No.:120462-46-6
- Lophanthoidin E
Catalog No.:BCN6092
CAS No.:120462-45-5
- Lophanthoidin B
Catalog No.:BCN6091
CAS No.:120462-42-2
- ER 27319 maleate
Catalog No.:BCC5914
CAS No.:1204480-26-1
- (±)-Vesamicol hydrochloride
Catalog No.:BCC6737
CAS No.:120447-62-3
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
- Verlukast
Catalog No.:BCC2035
CAS No.:120443-16-5
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- SRT3109
Catalog No.:BCC1965
CAS No.:1204707-71-0
- SRT3190
Catalog No.:BCC1966
CAS No.:1204707-73-2
- Desacetyldoronine
Catalog No.:BCN2105
CAS No.:120481-77-8
- Anastrozole
Catalog No.:BCC4370
CAS No.:120511-73-1
- 16-Nor-7,15-dioxodehydroabietic acid
Catalog No.:BCN7295
CAS No.:120591-53-9
- GLPG0634 analogue
Catalog No.:BCC6547
CAS No.:1206101-20-3
- GLPG0634
Catalog No.:BCC4145
CAS No.:1206161-97-8
- TC Mps1 12
Catalog No.:BCC7974
CAS No.:1206170-62-8
- Mofegiline hydrochloride
Catalog No.:BCC5412
CAS No.:120635-25-8
- 2beta-Acetoxyferruginol
Catalog No.:BCN7955
CAS No.:1206461-56-4
- 4,4'-Dihydroxy-3,3',9-trimethoxy-9,9'-epoxylignan
Catalog No.:BCN7017
CAS No.:1206464-65-4
- N-Benzylnaltrindole hydrochloride
Catalog No.:BCC6782
CAS No.:1206487-81-1
Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor D-1-methyl-tryptophan.[Pubmed:28228105]
J Hematol Oncol. 2017 Feb 23;10(1):56.
BACKGROUND: Although blinatumomab, a bispecific T cell engaging antibody, exhibits high clinical response rates in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia (B-ALL) and B cell non-Hodgkin's lymphoma (B-NHL), it still has some limitations because of its short half-life. Mesenchymal stromal cells (MSCs) represent an attractive approach for delivery of therapeutic agents to cancer sites owing to their tropism towards tumors, but their immunosuppression capabilities, especially induced by indoleamine 2,3-dioxygenase (IDO), should also be taken into consideration. METHODS: Human umbilical cord-derived MSCs (UC-MSCs) were genetically modified to secrete Tandab (CD3/CD19), a tetravalent bispecific tandem diabody with two binding sites for CD3 and two for CD19. The tropism of MSCs towards Raji cells in vitro was determined by migration assays, and the homing property of MSCs in vivo was analyzed with firefly luciferase-labeled MSCs (MSC-Luc) by bioluminescent imaging (BLI). The cytotoxicity of T cells induced by MSC-secreting Tandab (CD3/CD19) was detected in vitro and in vivo in combination with D-1-methyl-tryptophan (D-1MT), an IDO pathway inhibitor. RESULTS: The purified Tandab (CD3/CD19) was functional with high-binding capability both for CD3-positive cells and CD19-positive cells and was able to induce specific lysis of CD19-positive cell lines (Raji, Daudi, and BJAB) in the presence of T cells. Additionally, results from co-culture killing experiments demonstrated that Tandab (CD3/CD19) secreted from MSCs was also effective. Then, we confirmed that D-1MT could enhance the cytotoxicity of T cells triggered by MSC-Tandab through reversing T cell anergy with down-regulation of CD98 and Jumonji and restoring the proliferation capacity of T cells. Furthermore, MSC-Luc could selectively migrate to tumor site in a BALB/c nude mouse model with Raji cells. And mice injected with MSC-Tandab in combination with D-1MT significantly inhibited the tumor growth. CONCLUSIONS: These results suggest that UC-MSCs releasing Tandab (CD3/CD19) is an efficient therapeutic tool for the treatment of B cell lymphoma when combined with D-1MT.
Activation of the leukemia plasmacytoid dendritic cell line PMDC05 by Toho-1, a novel IDO inhibitor.[Pubmed:25075025]
Anticancer Res. 2014 Aug;34(8):4021-8.
BACKGROUND/AIM: Indoleamine-2,3-dioxygenase (IDO) is a rate-limiting enzyme for tryptophan metabolism and plays an immunosuppressive role. Antigen-presenting cells, when activated, increase the expression of IDO, which results in the suppression of subsequent immune reaction. A novel IDO inhibitor, Toho-1, was explored for its applicability to immunotherapy. MATERIALS AND METHODS: We investigated the effects of Toho-1 on antigen presentation and antigen-specific cytotoxic T-lymphocyte-inducing ability of leukemia plasmacytoid dendritic cell line PMDC05, which was established in our laboratory. RESULTS: While antigen presentation-associated molecules in PMDC05 cells were increased by stimulation with lipopolysaccharide and interferon-gamma, IDO mRNA and protein expression were also enhanced. Such treatment of PMDC05 cells in combination with Toho-1 enhanced the antigen-presenting and CTL-inducing ability of PMDC05 cells. CONCLUSION: These findings suggest the ability of Toho-1 to potentiate antigen-presenting cells and its applicability in immunotherapy of cancer.
The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells.[Pubmed:21625531]
PLoS One. 2011;6(5):e19823.
1-methyl-D-tryptophan (1-D-MT) is currently being used in clinical trials in patients with relapsed or refractory solid tumors with the aim of inhibiting indoleamine-2,3-dioxygenase (IDO)-mediated tumor immune escape. IDO is expressed in tumors and tumor-draining lymph nodes and degrades tryptophan (trp) to create an immunsuppressive micromilieu both by depleting trp and by accumulating immunosuppressive metabolites of the kynurenine (kyn) pathway. Here we show that proliferation of alloreactive T-cells cocultured with IDO1-positive human cancer cells paradoxically was inhibited by 1-D-MT. Surprisingly incubation with 1-D-MT increased kyn production of human cancer cells. Cell-free assays revealed that 1-D-MT did not alter IDO1 enzymatic activity. Instead, 1-D-MT induced IDO1 mRNA and protein expression through pathways involving p38 MAPK and JNK signalling. Treatment of cancer patients with 1-D-MT has transcriptional effects that may promote rather than suppress anti-tumor immune escape by increasing IDO1 in the cancer cells. These off-target effects should be carefully analyzed in the ongoing clinical trials with 1-D-MT.


