IDRA 21CAS# 22503-72-6 |
- NLG919
Catalog No.:BCC2325
CAS No.:1402836-58-1
- INCB024360 analogue
Catalog No.:BCC1647
CAS No.:914471-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22503-72-6 | SDF | Download SDF |
| PubChem ID | 3688 | Appearance | Powder |
| Formula | C8H9ClN2O2S | M.Wt | 232.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
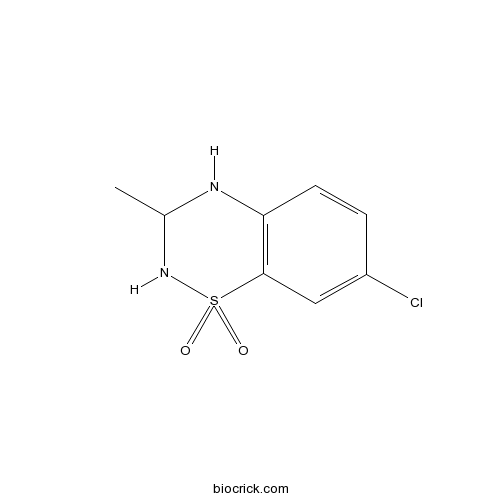
| Chemical Name | 7-chloro-3-methyl-3,4-dihydro-2H-1$l^{6},2,4-benzothiadiazine 1,1-dioxide | ||
| SMILES | CC1NC2=C(C=C(C=C2)Cl)S(=O)(=O)N1 | ||
| Standard InChIKey | VZRNTCHTJRLTMU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H9ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-5,10-11H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibits AMPA receptor desensitization and enhances cognition by a related mechanism. More able to cross the blood-brain barrier than cyclothiazide. |

IDRA 21 Dilution Calculator

IDRA 21 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2977 mL | 21.4887 mL | 42.9775 mL | 85.955 mL | 107.4437 mL |
| 5 mM | 0.8595 mL | 4.2977 mL | 8.5955 mL | 17.191 mL | 21.4887 mL |
| 10 mM | 0.4298 mL | 2.1489 mL | 4.2977 mL | 8.5955 mL | 10.7444 mL |
| 50 mM | 0.086 mL | 0.4298 mL | 0.8595 mL | 1.7191 mL | 2.1489 mL |
| 100 mM | 0.043 mL | 0.2149 mL | 0.4298 mL | 0.8595 mL | 1.0744 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-(1-hydroxy-2-(hydroxymethyl)-4-(4-octylphenyl)butan-2-yl)acetamide
Catalog No.:BCN1483
CAS No.:2249289-10-9
- Erigeside I
Catalog No.:BCN7172
CAS No.:224824-74-2
- Vardenafil HCl Trihydrate
Catalog No.:BCC2277
CAS No.:224785-90-4
- 18-Norabieta-8,11,13-trien-4-ol
Catalog No.:BCN5064
CAS No.:22478-65-5
- Gymnemagenin
Catalog No.:BCN7841
CAS No.:22467-07-8
- MLCK inhibitor peptide 18
Catalog No.:BCC5828
CAS No.:224579-74-2
- Benfotiamine
Catalog No.:BCC1415
CAS No.:22457-89-2
- Retapamulin
Catalog No.:BCC4837
CAS No.:224452-66-8
- Ginsenoside Rg1
Catalog No.:BCN1066
CAS No.:22427-39-0
- Bayogenin methyl ester
Catalog No.:BCN3722
CAS No.:22425-81-6
- 2,2'-Anhydro-5-methyluridine
Catalog No.:BCC8486
CAS No.:22423-26-3
- Incensole
Catalog No.:BCN3831
CAS No.:22419-74-5
- Falcarindiol
Catalog No.:BCN5065
CAS No.:225110-25-8
- Cathepsin Inhibitor 1
Catalog No.:BCC4896
CAS No.:225120-65-0
- 8-Hydroxy-9,10-diisobutyryloxythymol
Catalog No.:BCN7786
CAS No.:22518-08-7
- CTU Guanamine
Catalog No.:BCC8921
CAS No.:22535-90-6
- Ocotillone
Catalog No.:BCN5066
CAS No.:22549-21-9
- Robustine
Catalog No.:BCN6653
CAS No.:2255-50-7
- Isocryptotanshinone
Catalog No.:BCN2499
CAS No.:22550-15-8
- Bisabolol Oxide A
Catalog No.:BCC8133
CAS No.:22567-36-8
- Zeorin
Catalog No.:BCN5067
CAS No.:22570-53-2
- Symphytine
Catalog No.:BCN1975
CAS No.:22571-95-5
- PAR 4 (1-6)
Catalog No.:BCC3956
CAS No.:225779-44-2
- Epifriedelanol acetate
Catalog No.:BCN5068
CAS No.:2259-07-6
The diazoxide derivative IDRA 21 enhances ischemic hippocampal neuron injury.[Pubmed:9585363]
Ann Neurol. 1998 May;43(5):664-9.
The diazoxide derivative IDRA 21 and other positive modulators of (AMPA)-type glutamate receptors are considered potential memory-enhancing agents. However, AMPA receptor activation contributes to CA1 hippocampal neuron damage from global ischemia in rodents, raising the possibility that 7-chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21) or drugs with similar actions may worsen ischemic neuronal injury. Here we demonstrate that glutamate plus IDRA 21 kills cultured rat hippocampal neurons by AMPA receptor activation, and, in vivo, 12 and 24 mg/kg of IDRA 21 given orally increases CA1 neuron loss produced by 10 minutes of global ischemia. Treating patients with drugs that potentiate AMPA receptor activation will have to consider these potential effects, particularly when coexistent with conditions in which excessive activation of AMPA receptors may occur (eg, stroke, seizures).
IDRA-21, a positive AMPA receptor modulator, inhibits synaptic and extrasynaptic NMDA receptor mediated events in cultured cerebellar granule cells.[Pubmed:15111017]
Neuropharmacology. 2004 Jun;46(8):1105-13.
IDRA-21 (7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide) reduces alpha-amino-3-hydroxy-5-methylisoxazolepropionic acid (AMPA) receptors desensitisation in vitro and restores learning and cognitive impairment in vivo. In this study, we show that in cerebellar granule cells (CGCs) in culture IDRA-21 reduces N-methyl-d-aspartate receptor (NMDAR) whole-cell currents. The effect is neither competitive nor voltage-dependent. The reduction of NMDA currents is stronger at low glycine concentrations suggesting an interaction with this site. IDRA-21 shortens miniature excitatory postsynaptic currents mediated by NMDARs (NMDA-mEPSCs) in CGCs grown in low potassium with no effect on peak amplitudes. By using fast glutamate application onto CGCs nucleated patches, we found that IDRA-21 decreases both decay time constant and amplitude of the current. Experiments performed on recombinant NMDAR expressed in HEK 293 cells showed that IDRA-21 was more effective on NR1a-NR2B than NR1a-NR2A receptors highlighting a subunit selectivity of the drug. Our findings make light on a novel target for IDRA-21: NMDA receptors function is negatively modulated and the different action at the level of extrasynaptic and synaptic receptors could be ascribed to a partial selectivity for NR2B subunits.
The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys.[Pubmed:14654093]
Neuropharmacology. 2004 Jan;46(1):10-22.
IDRA 21, a positive allosteric modulator of the glutamate AMPA receptor, produced a concentration-dependent inhibition of glutamate-induced inactivation of membrane currents in recombinant HEK 293 (human embryonic kidney) cells stably transfected with human GluR1/2 flip receptors. IDRA 21 doubled the charge transfer at a concentration of 70 microM, suggesting that this compound can facilitate excitatory neurotransmission via GluR 1/2 receptors. We next sought to exploit this mechanism of action by examining the drug as a potential cognition-enhancing agent in non-human primates. Oral administration of IDRA 21 produced a highly significant improvement in the performance of a delayed matching-to-sample (DMTS) task by young adult rhesus monkeys. The pattern of task improvement over the dose range 0.15-10 mg/kg was maintained to 48 hr after the single dose administration. For sessions run after administration of the individualized Best Dose of IDRA 21, task accuracy for Long delay (most difficult) trials was increased by 34% of vehicle. Animals were randomly assigned fixed doses of IDRA 21 to determine whether the positive mnemonic response could be maintained. The repeated doses were separated by 3 days, thus allowing for potential cumulative effects. IDRA 21 produced a gradual increase in task accuracy that was maintained on average above vehicle performance levels over an intermittent dosing schedule during a total period of 3 weeks. A separate group of aged monkeys (>20 y) were, as a group, impaired (during vehicle testing) in DMTS performance efficiency relative to the young cohort. IDRA 21 also improved task accuracy by aged rhesus monkeys over the same dose range, but the responses were not as robust as those exhibited by young animals. Aged subjects also appeared to be more individually sensitive to drug dose, and they exhibited shorter task latencies than did the young group. Despite these differences, when the individualized Best Doses were considered, IDRA 21 produced a robust increase in DMTS accuracy of up to 18% of vehicle for trials associated with Medium delay intervals. For both study groups, no obvious untoward effects of IDRA 21 were noted. These findings support the use of AMPA modulators like IDRA 21 in the treatment of cognitive/memory disorders, including those associated with aging. They also indicate that the drug is associated with long-term effects that could limit dosing regimens to one dose every two or three days. The nature of the protracted mnemonic effects produced by the compound remains to be elucidated.
The effects of huperzine A and IDRA 21 on visual recognition memory in young macaques.[Pubmed:21185313]
Neuropharmacology. 2011 Jun;60(7-8):1262-8.
Nootropic agents or cognitive enhancers are purported to improve mental functions such as cognition, memory, or attention. The aim of our study was to determine the effects of two possible cognitive enhancers, huperzine A and IDRA 21, in normal young adult monkeys performing a visual memory task of varying degrees of difficulty. Huperzine A is a reversible acetylcholinesterase (AChE) inhibitor, its administration results in regionally specific increases in acetylcholine levels in the brain. In human clinical trials, Huperzine A resulted in cognitive improvement in patients with mild to moderate form of Alzheimer's disease (AD) showing its potential as a palliative agent in the treatment of AD. IDRA 21 is a positive allosteric modulator of glutamate AMPA receptors. It increases excitatory synaptic strength by attenuating rapid desensitization of AMPA receptors and may thus have beneficial therapeutic effects to ameliorate memory deficits in patients with cognitive impairments, including AD. The present study evaluated the effects of the two drugs in normal, intact, young adult monkeys to determine whether they can result in cognitive enhancement in a system that is presumably functioning optimally. Six young pigtail macaques (Macaca nemestrina) were trained on delayed non-matching-to-sample task, a measure of visual recognition memory, up to criterion of 90% correct responses on each of the four delays (10s, 30s, 60s, and 90s). They were then tested on two versions of the task: Task 1 included the four delays intermixed within a session and the monkeys performed it with the accuracy of 90%. Task 2 included, in each of 24 trials, a list of six objects presented in succession. Two objects from the list were then presented for choice paired with novel objects and following two of the four delays intermixed within a session. This task with a higher mnemonic demand yielded an average performance of 64% correct. Oral administration of huperzine A did not significantly affect the monkeys' performance on either task. However, a significant negative correlation was found between the baseline performance on each delay and the change in performance under huperzine A, suggesting that under conditions in which the subjects were performing poorly (55-69%), the drug resulted in improved performance, whereas no improvement was obtained when the baseline was close to 90%. In fact, when the subjects were performing very well, huperzine A tended to reduce the performance accuracy, indicating that in a system that functions optimally, the increased availability of acetylcholine does not improve performance or memory, especially when the animals are close to the maximum performance. In contrast, oral administration of IDRA 21 significantly improved performance on Task 2, especially on the longest delay. This finding supports the potential use of this drug in treatment of cognitive and memory disorders. This article is part of a Special Issue entitled 'Trends in neuropharmacology: in memory of Erminio Costa'.
7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide: a partial modulator of AMPA receptor desensitization devoid of neurotoxicity.[Pubmed:9192690]
Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):7053-8.
In cerebellar granule neurons of neonatal rats micromolar concentrations of 7-chloro-3-methyl-3,4-dihydro-2H-1,2, 4-benzothiadiazine S,S-dioxide (IDRA-21) and cyclothiazide, two negative modulators of the spontaneous agonist-dependent rapid desensitization of alpha-amino-3-hydroxy-5-methylisoxazolepropionic acid (AMPA)-gated ion channels, facilitate AMPA receptor function by increasing the content of free cytosolic Ca2+ as measured by single-cell fura-2 acetoxymethyl ester (Fura-2) Ca2+-dependent fluorescence and intracellular Na+ measured with the sodium-binding bezofuran isophthalate acetoxymethyl ester fluorescence indicator. IDRA-21 increases intracellular Na+ transient with a threshold (5 microM) that is approximately 10 times higher and has an intrinsic activity significantly lower than that of cyclothiazide. By virtue of its low intrinsic activity, IDRA-21 elicits a free cytosolic Ca2+ transient increase that is shorter lasting than that elicited by cyclothiazide even when the drug is left in contact with cultured granule cells for several minutes. Additionally, while dose dependently, 5-25 microM cyclothiazide in the presence of AMPA is highly neurotoxic, IDRA-21 (up to 100 microM) is devoid of neurotoxicity. The neurotoxicity elicited by cyclothiazide persists in the presence of dizocilpine (an antagonist of N-methyl-D-aspartate-selective glutamate receptors) but is blocked by 2,3-dihydroxy-6-nitrosulfamoylbenzo[f]quinoxaline (a competitive AMPA receptor antagonist) and the 1-(aminophenyl)-4-methyl-7, 8-methylendioxy-5H-2,3-benzodiazepine (GYKI 52466; a noncompetitive AMPA receptor antagonist). Since the doses of IDRA-21 that enhance cognitive processes in rats and monkeys are several orders of magnitude lower than those required to elicit marginal neurotoxicity in cultured neurons, it can be surmised that IDRA-21 is a potent cognition-enhancing drug virtually devoid of neurotoxic liability because it acts as a partial negative allosteric modulator of AMPA receptor desensitization.
7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys.[Pubmed:7644474]
Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7667-71.
We report here on the ability of IDRA 21 and aniracetam, two negative allosteric modulators of glutamate-induced DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor desensitization, to attenuate alprazolam-induced learning deficit in patas monkeys working in a complex behavioral task. In one component of a multiple schedule (repeated acquisition or "learning"), patas monkeys acquired a different four-response chain each session by responding sequentially on three keys in the presence of four discriminative stimuli (geometric forms or numerals). In the other component (performance) the four-response chain was the same each session. The response chain in each component was maintained by food presentation under a fixed-ratio schedule. When alprazolam (0.1 or 0.32 mg/kg p.o.) was administered alone, this full allosteric modulator of gamma-aminobutyric acid type A (GABAA) receptors produced large decreases in the response rate and accuracy in the learning component of the task. IDRA 21 (3 or 5.6 mg/kg p.o.) and aniracetam (30 mg/kg p.o.) administered 60 min before alprazolam, having no effect when given alone, antagonized the large disruptive effects of alprazolam on learning. From dose-response studies, it can be estimated that IDRA 21 is approximately 10-fold more potent than aniracetam in antagonizing alprazolam-induced learning deficit. We conclude that IDRA 21, a chemically unrelated pharmacological congener of aniracetam, improves learning deficit induced in patas monkeys by the increase of GABAergic tone elicited by alprazolam. Very likely IDRA 21 exerts its behavioral effects by antagonizing AMPA receptor desensitization.
7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization.[Pubmed:7815345]
J Pharmacol Exp Ther. 1995 Jan;272(1):300-9.
7-Chloro-3-Methyl-3-4-Dihydro-2H-1,2,4 Benzothiadiazine S,S Dioxide (IDRA 21), which attenuates the rapid autodesensitization of DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA)-selective glutamate receptors and increases excitatory synaptic strength, improves cognition (learning and memory), as revealed by its ability to improve performance in water maze and passive avoidance tests in rats. Normal rats trained to (15-20 sec) reach the exit platform rapidly in a water maze that included four incorrect choices were given oral IDRA 21 (4-120 mumol/kg) or vehicle and then exposed to a delayed retention trial in a maze that included seven incorrect choices. In this retention trial, the IDRA 21-treated rats performed considerably better than those that received the vehicle. Moreover, oral IDRA 21 (ED50 = 7.6 microM) attenuated the performance impairment induced by the AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo (F) quinoxaline in the water maze test. In this test and in a passive avoidance test, the performance impairment elicited by alprazolam, a full allosteric modulator at gamma-aminobutyric acid-A receptors, or by scopolamine, a competitive muscarinic receptor antagonist, was also reduced by oral administration of IDRA 21 (ED50 = 13 and 108 mumol/kg, against alprazolam and scopolamine, respectively); in all these tests, IDRA 21 was 20- to 30-fold more potent than aniracetam. Because IDRA 21 is a racemic molecule; the two stereoisomers were isolated and studied behaviorally. Only the (+) form was found to be behaviorally active. These results indicate that IDRA 21 given orally to rats presumably crosses the blood-brain barrier and acts stereoselectively on specific receptors that were operative during this behavioral procedure. Because the activity of IDRA 21 on rat cognition tests appears to be related to its ability to potentiate AMPA-activated currents, one can suggest that IDRA 21 improves cognition by acting on a stereoselective site of AMPA receptor that is operative in attenuating the rapid autodesensitization of these receptors.


