HypaphorineCAS# 487-58-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 487-58-1 | SDF | Download SDF |
| PubChem ID | 442106 | Appearance | Powder |
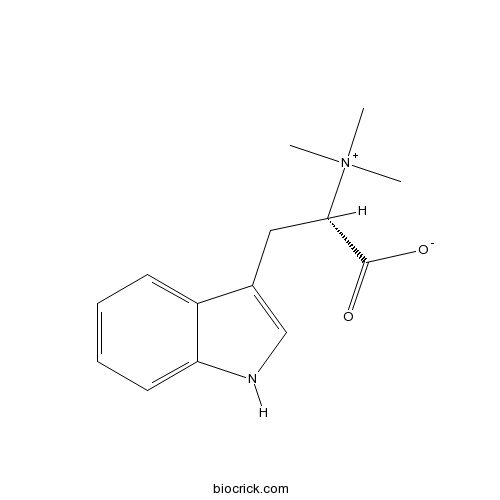
| Formula | C14H18N2O2 | M.Wt | 246.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-3-(1H-indol-3-yl)-2-(trimethylazaniumyl)propanoate | ||
| SMILES | C[N+](C)(C)C(CC1=CNC2=CC=CC=C21)C(=O)[O-] | ||
| Standard InChIKey | AOHCBEAZXHZMOR-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C14H18N2O2/c1-16(2,3)13(14(17)18)8-10-9-15-12-7-5-4-6-11(10)12/h4-7,9,13,15H,8H2,1-3H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hypaphorine is an indole-3-acetic acid antagonist which specifically compete with indole-3-acetic acid in binding to the indole-3-acetic acid-binding site in plant peroxidases.Hypaphorine and endogenous indole-3-acetic acid counteract in controlling root hair elongation. |
| In vitro | Root hair elongation is inhibited by hypaphorine, the indole alkaloid from the ectomycorrhizal fungus Pisolithus tinctorius, and restored by indole-3-acetic acid.[Pubmed: 11089686 ]Planta. 2000 Oct;211(5):722-8.Hypaphorine, the major indolic compound isolated from the ectomycorrhizal fungus Pisolithus tinctorius, controls the elongation rate of root hairs. |
| In vivo | Hypaphorine is present in human milk in association with consumption of legumes.[Pubmed: 23855762]J Agric Food Chem. 2013 Aug 7;61(31):7654-60.In metabolomic analysis of human milk amines, we found a previously unidentified compound. This was tentatively identified as Hypaphorine, an indole alkaloid composed of tryptophan and three methyls, and with neurological and glucose-lowering effects in rodents. |
| Kinase Assay | A fungal auxin antagonist, hypaphorine prevents the indole-3-acetic acid-dependent irreversible inactivation of horseradish peroxidase: inhibition of Compound III-mediated formation of P-670.[Pubmed: 12056802]Biochem Biophys Res Commun. 2002 Jun 14;294(3):553-9.Hypaphorine, an indolic alkaloid from an ectomycorrhizal fungus is a putative antagonist of indole-3-acetic acid (IAA) known to inhibit the effect of IAA in growing roots of Eucalyptus seedling. Previously we have used horseradish peroxidase-C (HRP) as a sensitive reporter of IAA-binding to the IAA-binding domain, and reported that Hypaphorine specifically inhibits the HRP-catalyzed superoxide generation coupled to oxidation of IAA [Kawano et al., Biochem. Biophys. Res. Commun. 288]. Since binding of IAA to the auxin-binding domain is the key step required for IAA oxidation by HRP, it was assumed that the inhibitory effect of Hypaphorine is due to its competitive binding to the auxin-binding domain in HRP. |
| Structure Identification | Planta. 2003 Dec;218(2):217-25.Hypaphorine, an indole-3-acetic acid antagonist delivered by the ectomycorrhizal fungus Pisolithus tinctorius, induces reorganisation of actin and the microtubule cytoskeleton in Eucalyptus globulus ssp bicostata root hairs.[Pubmed: 14504925]Hypaphorine, an indole alkaloid from the ectomycorrhizal fungus Pisolithus tinctorius Coker & Couch., counteracts indole-3-acetic acid (IAA) activity and controls the rate of root hair elongation in Eucalyptus globulus ssp. bicostata. |

Hypaphorine Dilution Calculator

Hypaphorine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Butein
Catalog No.:BCN5592
CAS No.:487-52-5
- Ononetin
Catalog No.:BCC6367
CAS No.:487-49-0
- Phillyrin
Catalog No.:BCN1096
CAS No.:487-41-2
- Phillygenin
Catalog No.:BCN2653
CAS No.:487-39-8
- Pinoresinol
Catalog No.:BCN5591
CAS No.:487-36-5
- Syringaresinol
Catalog No.:BCN3042
CAS No.:487-35-4
- Scopoline
Catalog No.:BCN1942
CAS No.:487-27-4
- Elemicin
Catalog No.:BCN2818
CAS No.:487-11-6
- Citropten
Catalog No.:BCN4831
CAS No.:487-06-9
- Cyclocalopin A
Catalog No.:BCN5587
CAS No.:486430-94-8
- O-Acetylcyclocalopin A
Catalog No.:BCN5586
CAS No.:486430-93-7
- CIQ
Catalog No.:BCC7862
CAS No.:486427-17-2
- Kainic acid
Catalog No.:BCC6572
CAS No.:487-79-6
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- SNAP 94847 hydrochloride
Catalog No.:BCC7658
CAS No.:487051-12-7
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
- Thesinine
Catalog No.:BCN1990
CAS No.:488-02-8
- 3-Methylcatechol
Catalog No.:BCN3925
CAS No.:488-17-5
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- vibo-Quercitol
Catalog No.:BCN5594
CAS No.:488-76-6
- D-Ribitol-5-phosphate
Catalog No.:BCC4838
CAS No.:35320-17-3
Hypaphorine is present in human milk in association with consumption of legumes.[Pubmed:23855762]
J Agric Food Chem. 2013 Aug 7;61(31):7654-60.
In metabolomic analysis of human milk amines, we found a previously unidentified compound. This was tentatively identified as Hypaphorine, an indole alkaloid composed of tryptophan and three methyls, and with neurological and glucose-lowering effects in rodents. Hypaphorine identity was confirmed by Hypaphorine synthesis, and then a fluorometric method was developed to quantify Hypaphorine in milk and foods. Using dietary records, we identified peanut products as probable sources of Hypaphorine. Milk from 24 lactating women showed widely varying Hypaphorine, with a mean +/- SD 0.34 +/- 0.33 muM, and the highest concentration of 1.24 muM. Peanuts showed high Hypaphorine of 70 mug/g compared to 60 and 100 mug/g in dried chickpeas and lentils. Dietary challenge in lactating women with Hypaphorine-rich foods demonstrated transfer of Hypaphorine into milk with Hypaphorine appearance peaking 5-18 h after consumption and prolonged disappearance indicative of slow excretion or metabolism. The potential functional roles of Hypaphorine in human nutrition remain to be addressed.
Hypaphorine, an indole-3-acetic acid antagonist delivered by the ectomycorrhizal fungus Pisolithus tinctorius, induces reorganisation of actin and the microtubule cytoskeleton in Eucalyptus globulus ssp bicostata root hairs.[Pubmed:14504925]
Planta. 2003 Dec;218(2):217-25.
Hypaphorine, an indole alkaloid from the ectomycorrhizal fungus Pisolithus tinctorius Coker & Couch., counteracts indole-3-acetic acid (IAA) activity and controls the rate of root hair elongation in Eucalyptus globulus ssp. bicostata. The present investigation shows that Hypaphorine changes cytoskeletal organisation in elongating root hairs of the host. The actin cytoskeleton was investigated by two different fixation and labelling procedures, which gave similar results. In control root hairs, actin organisation was characterised by (i) an actin cap at the very tip region, (ii) a subapical region with reduced labelling and containing fine actin filaments, and (iii) axial bundles of actin filaments running from the subapical part to the base of the root hair. In the Hypaphorine-treated root hairs no actin cap was distinguished. The fine actin filaments occurring in the subapical region were replaced by a few thick actin filament bundles that extended from the subapical region toward the root hair tip. In the Hypaphorine-treated hairs the total number of actin filament bundles along most of the root hair length was significantly reduced, presumably due to aggregation of pre-existing actin filaments. The first signs of alteration to the cytoskeleton could be detected as soon as 15 min after Hypaphorine treatment. In Hypaphorine-treated, but not in control root hairs, a patch of aggregated microtubules regularly occurred at a distance of approximately 10 microm from the tip, possibly as a consequence of changes induced by Hypaphorine in the actin cytoskeleton. The Hypaphorine-induced aggregations in the actin and microtubule cytoskeletons could stabilise the structure of cytoskeletal elements, which in turn could hinder the vesicle delivery at the tip necessary for elongation. Such cytoskeletal alterations may be a consequence of the antagonism between IAA and Hypaphorine. The latter view was supported by restoration of the actin cytoskeleton in Hypaphorine-treated root hairs by IAA application.
Root hair elongation is inhibited by hypaphorine, the indole alkaloid from the ectomycorrhizal fungus Pisolithus tinctorius, and restored by indole-3-acetic acid.[Pubmed:11089686]
Planta. 2000 Oct;211(5):722-8.
Hypaphorine, the major indolic compound isolated from the ectomycorrhizal fungus Pisolithus tinctorius, controls the elongation rate of root hairs. At inhibitory concentrations (100 microM), Hypaphorine induced a transitory swelling of root hair tips of Eucalyptus globulus Labill. ssp. bicostata. When the polar tip growth resumed, a characteristic deformation was still visible on elongating hairs. At higher Hypaphorine concentrations (500 microM and greater), root hair elongation stopped, only 15 min after application. However, root hair initiation from trichoblasts was not affected by Hypaphorine. Hypaphorine activity could not be mimicked by related molecules such as indole-3-acetic acid (IAA) or tryptophan. While IAA had no activity on root hair elongation, IAA was able to restore the tip growth of root hairs following inhibition by Hypaphorine. These results suggest that Hypaphorine and endogenous IAA counteract in controlling root hair elongation. During ectomycorrhiza development, the absence of root hairs might be due in part to fungal release of molecules, such as Hypaphorine, that inhibit the elongation of root hairs.
A fungal auxin antagonist, hypaphorine prevents the indole-3-acetic acid-dependent irreversible inactivation of horseradish peroxidase: inhibition of Compound III-mediated formation of P-670.[Pubmed:12056802]
Biochem Biophys Res Commun. 2002 Jun 14;294(3):553-9.
Hypaphorine, an indolic alkaloid from an ectomycorrhizal fungus is a putative antagonist of indole-3-acetic acid (IAA) known to inhibit the effect of IAA in growing roots of Eucalyptus seedling. Previously we have used horseradish peroxidase-C (HRP) as a sensitive reporter of IAA-binding to the IAA-binding domain, and reported that Hypaphorine specifically inhibits the HRP-catalyzed superoxide generation coupled to oxidation of IAA [Kawano et al., Biochem. Biophys. Res. Commun. 288]. Since binding of IAA to the auxin-binding domain is the key step required for IAA oxidation by HRP, it was assumed that the inhibitory effect of Hypaphorine is due to its competitive binding to the auxin-binding domain in HRP. Here, we obtained further evidence in support of our assumption that Hypaphorine specifically inhibits binding of IAA to HRP. In this study, HRP arrested at the temporal inactive form known as Compound III was used as a sensitive indicator for binding of IAA to HRP. Addition of IAA to the preformed Compound III resulted in rapid decreases in absorption maxima at 415, 545, and 578 nm characteristic to Compound III, and in turn a rapid increase in absorption maximum at 670 nm representing the formation of P-670, the irreversibly inactivated form of hemoproteins, was induced. In contrast, the IAA-dependent irreversible inactivation of HRP was inhibited in the presence of Hypaphorine. In addition, the mode of interaction between IAA and Hypaphorine was determined to be competitive inhibition, further confirming that Hypaphorine is an IAA antagonist which specifically compete with IAA in binding to the IAA-binding site in plant peroxidases.


