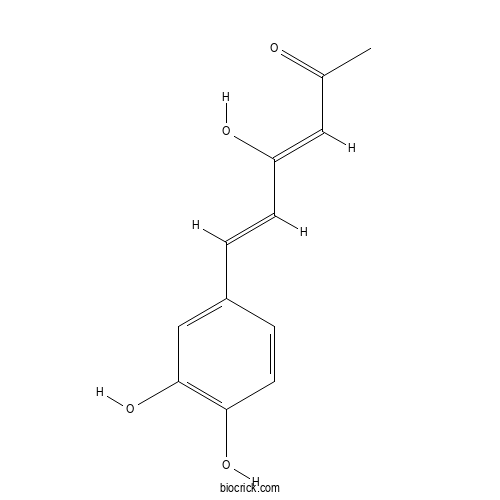
HispolonCAS# 173933-40-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 173933-40-9 | SDF | Download SDF |
| PubChem ID | 10082188.0 | Appearance | Powder |
| Formula | C12H12O4 | M.Wt | 220.22 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3Z,5E)-6-(3,4-dihydroxyphenyl)-4-hydroxyhexa-3,5-dien-2-one | ||
| SMILES | CC(=O)C=C(C=CC1=CC(=C(C=C1)O)O)O | ||
| Standard InChIKey | QDVIEIMMEUCFMW-QXYPORFMSA-N | ||
| Standard InChI | InChI=1S/C12H12O4/c1-8(13)6-10(14)4-2-9-3-5-11(15)12(16)7-9/h2-7,14-16H,1H3/b4-2+,10-6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hispolon Dilution Calculator

Hispolon Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5409 mL | 22.7046 mL | 45.4091 mL | 90.8183 mL | 113.5228 mL |
| 5 mM | 0.9082 mL | 4.5409 mL | 9.0818 mL | 18.1637 mL | 22.7046 mL |
| 10 mM | 0.4541 mL | 2.2705 mL | 4.5409 mL | 9.0818 mL | 11.3523 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9082 mL | 1.8164 mL | 2.2705 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.4541 mL | 0.9082 mL | 1.1352 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-methoxy-bispyranoxanthone
Catalog No.:BCX1166
CAS No.:115713-10-5
- Aucubigenin
Catalog No.:BCX1165
CAS No.:64274-28-8
- Coronafacic acid
Catalog No.:BCX1164
CAS No.:62251-98-3
- Coronatine
Catalog No.:BCX1163
CAS No.:62251-96-1
- Soyasaponin Ae
Catalog No.:BCX1162
CAS No.:117230-34-9
- Soyasaponin Af
Catalog No.:BCX1161
CAS No.:117230-32-7
- Zingiberene
Catalog No.:BCX1160
CAS No.:495-60-3
- Neotheaflavin
Catalog No.:BCX1159
CAS No.:36451-14-6
- Neolinustatin
Catalog No.:BCX1158
CAS No.:72229-42-6
- Kadsurenone
Catalog No.:BCX1157
CAS No.:95851-37-9
- Proprotogracillin
Catalog No.:BCX1156
CAS No.:78229-03-5
- Pyroside
Catalog No.:BCX1155
CAS No.:10338-88-2
- 25(R)-3β,17α-Dihydroxy-5α-spirostan-6-one 3-O-α-D-rhamnopyranosyl-(1→2)-β-D-glucopyranoside
Catalog No.:BCX1168
CAS No.:143051-94-9
- Guattegaumerine
Catalog No.:BCX1169
CAS No.:21446-35-5
- Ganolactone A
Catalog No.:BCX1170
CAS No.:173268-82-1
- Cyaonoside B
Catalog No.:BCX1171
CAS No.:51161-58-1
- Tirotundin 3-O-methyl ether
Catalog No.:BCX1172
CAS No.:1454840-36-8
- 15-Oxospiramilactone
Catalog No.:BCX1173
CAS No.:1053172-87-4
- 7-Oxo-ganoderic acid Z2
Catalog No.:BCX1174
CAS No.:1446104-52-4
- Aloenin B
Catalog No.:BCX1175
CAS No.:106533-41-9
- Glucovanillin
Catalog No.:BCX1176
CAS No.:494-08-6
- Halofuginone hydrobromide
Catalog No.:BCX1177
CAS No.:64924-67-0
- 2-Hydroxypinocembrin
Catalog No.:BCX1178
CAS No.:40489-17-6
- 11-Oxo-ganoderic acid DM
Catalog No.:BCX1179
CAS No.:1408244-15-4
Hispolon inhibits neuronal ferroptosis by promoting the expression of Nrf-2.[Pubmed:38305125]
Neuroreport. 2024 Mar 6;35(4):242-249.
Research has shown that neuronal ferroptosis is associated with various central nervous system diseases, including Parkinson's disease, acute brain injury, and spinal cord injury. Inhibiting neuronal ferroptosis can greatly alleviate the progression of these diseases. However, there is currently a lack of effective drugs to inhibit neuronal ferroptosis. In this study, we pretreated neuronal cells with Hispolon and subsequently induced a neuronal ferroptosis model using Erastin. We further assessed the changes in the protein expression levels of SLC7A11, GPX4, ACSL4, Nrf-2, and HO-1 using Western blot and immunofluorescence techniques. Additionally, we measured the intracellular levels of Fe2+, GSH, and MDA using relevant assay kits. The research findings revealed that after Hispolon treatment, the expression of the pro-ferroptosis protein ACSL4 decreased, while the expression of the ferroptosis-regulating proteins GPX4 and SLC7A11 increased. Moreover, the use of an Nrf-2-specific inhibitor was able to reverse the effects of Hispolon as mentioned above. In this study, we discovered that Hispolon can promote the expression of Nrf-2 and inhibit the occurrence of neuronal ferroptosis induced by Erastin.
Methoxyhispolon Methyl Ether, a Hispolon Analog, Thwarts the SRC/STAT3/BCL-2 Axis to Provoke Human Triple-Negative Breast Cancer Cell Apoptosis In Vitro.[Pubmed:37893115]
Biomedicines. 2023 Oct 10;11(10):2742.
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer with few treatment options. A promising TNBC treatment approach is targeting the oncogenic signaling pathways pivotal to TNBC initiation and progression. Deregulated activation of signal transducer and activator of transcription 3 (STAT3) is fundamental to driving TNBC malignant transformation, highlighting STAT3 as a promising TNBC therapeutic target. MethoxyHispolon Methyl Ether (MHME) is an analog of Hispolon, an anti-cancer polyphenol found in the medicinal mushroom Phellinus linteus. Still, MHME's anti-cancer effects and mechanisms remain unknown. Herein, we present the first report about MHME's anti-TNBC effect and its action mechanism. We first revealed that MHME is proapoptotic and cytotoxic against human TNBC cell lines HS578T, MDA-MB-231, and MDA-MB-463 and displayed a more potent cytotoxicity than Hispolon's. Mechanistically, MHME suppressed both constitutive and interleukin 6 (IL-6)-induced activation of STAT3 represented by the extent of tyrosine 705-phosphorylated STAT3 (p-STAT3). Notably, MHME-evoked apoptosis and clonogenicity impairment were abrogated in TNBC cells overexpressing a dominant-active mutant of STAT3 (STAT3-C); supporting the blockade of STAT3 activation is an integral mechanism of MHME's cytotoxic action on TNBC cells. Moreover, MHME downregulated BCL-2 in a STAT3-dependent manner, and TNBC cells overexpressing BCL-2 were refractory to MHME-induced apoptosis, indicating that BCL-2 downregulation is responsible for MHME's proapoptotic effect on TNBC cells. Finally, MHME suppressed SRC activation, while v-src overexpression rescued p-STAT3 levels and downregulated apoptosis in MHME-treated TNBC cells. Collectively, we conclude that MHME provokes TNBC cell apoptosis through the blockade of the SRC/STAT3/BCL-2 pro-survival axis. Our findings suggest the potential of applying MHME as a TNBC chemotherapy agent.
Blockade of the SRC/STAT3/BCL-2 Signaling Axis Sustains the Cytotoxicity in Human Colorectal Cancer Cell Lines Induced by Dehydroxyhispolon Methyl Ether.[Pubmed:37760971]
Biomedicines. 2023 Sep 13;11(9):2530.
Colorectal cancer (CRC) is the third most prevalent human cancer globally. 5-Fluorouracil (5-FU)-based systemic chemotherapy is the primary strategy for advanced CRC treatment, yet is limited by poor response rate. Deregulated activation of signal transducer and activator of transcription 3 (STAT3) is fundamental to driving CRC malignant transformation and a poor prognostic marker for CRC, underscoring STAT3 as a promising CRC drug target. DehydroxyHispolon methyl ether (DHME) is an analog of Hispolon, an anticancer polyphenol abundant in the medicinal mushroom Phellinus linteus. Previously, we have established DHME's cytotoxic effect on human CRC cell lines by eliciting apoptosis through the blockade of WNT/beta-catenin signaling, a preeminent CRC oncogenic pathway. Herein, we unraveled that compared with 5-FU, DHME is a more potent killer of CRC cells while being much less toxic to normal colon epithelial cells. DHME suppressed both constitutive and interleukin 6 (IL-6)-induced STAT3 activation represented by tyrosine 705 phosphorylation of STAT3 (p-STAT3 (Y705)); notably, DHME-induced CRC apoptosis and clonogenicity limitation were abrogated by ectopic expression of STAT3-C, a dominant-active STAT3 mutant. Additionally, we proved that BCL-2 downregulation caused by DHME-mediated STAT3 blockage is responsible for DHME-induced CRC cell apoptosis. Lastly, DHME inhibited SRC activation, and v-src overexpression restored p-STAT3 (Y705) levels along with lowering the levels of apoptosis in DHME-treated CRC cells. We conclude DHME provokes CRC cell apoptosis by blocking the SRC/STAT3/BCL-2 axis besides thwarting WNT/beta-catenin signaling. The notion that DHME targets two fundamental CRC signaling pathways underpins the potential of DHME as a CRC chemotherapy agent.
Augmentation of Docetaxel-Induced Cytotoxicity in Human PC-3 Androgen-Independent Prostate Cancer Cells by Combination With Four Natural Apoptosis-Inducing Anticancer Compounds.[Pubmed:37292146]
Nat Prod Commun. 2023 May;18(5):10.1177/1934578x231175323.
Docetaxel (DTX) is the treatment of choice for metastatic castration-resistant prostate cancer. However, developing drug resistance is a significant challenge for achieving effective therapy. This study evaluated the anticancer and synergistic effects on DTX of four natural compounds (calebin A, 3(')-hydroxypterostilbene, Hispolon, and tetrahydrocurcumin) using PC-3 androgen-resistant human prostate cancer cells. We utilized the CellTiter-Glo((R)) luminescent cell viability assay and human PC-3 androgen-independent prostate cancer cells to determine the antiproliferative effects of the four compounds alone and combined with DTX. Cytotoxicity to normal human prostate epithelial cells was tested in parallel using normal immortalized human prostate epithelial cells (RWPE-1). We used cell imaging and quantitative caspase-3 activity to determine whether these compounds induce apoptosis. We also measured the capacity of each drug to inhibit TNF-alpha-induced NF-kB using a colorimetric assay. Our results showed that all four natural compounds significantly augmented the toxicity of DTX to androgen-resistant PC-3 prostate cancer cells at IC(50). Interestingly, when used alone, each of the four compounds had a higher cytotoxic activity to PC-3 than DTX. Mechanistically, these compounds induced apoptosis, which we confirmed by cell imaging and caspase-3 colorimetric assays. Further, when used either alone or combined with DTX, the four test compounds inhibited TNF-alpha-induced NF-kB production. More significantly, the cytotoxic effects on normal immortalized human prostate epithelial cells were minimal and non-significant, suggesting prostate cancer-specific effects. In conclusion, the combination of DTX with the four test compounds could effectively enhance the anti-prostate cancer activity of DTX. This combination has the added value of reducing the DTX effective concentration. We surmise that calebin A, 3(')-hydroxypterostilbene, Hispolon, and tetrahydrocurcumin were all excellent drug candidates that produced significant antiproliferative activity when used alone and synergistically enhanced the anticancer effect of DTX. Further in vivo studies using animal models of prostate cancer are needed to confirm our in vitro findings.
Systematic Characterization of Active Antitumor Constituents from the Shaggy Bracket Medicinal Mushroom Inonotus hispidus (Agaricomycetes) by UPLC-Q-TOF/MS and Network Pharmacology.[Pubmed:37017661]
Int J Med Mushrooms. 2023;25(3):47-62.
Inonotus hispidus is a well-known medicinal fungus and has been used in the treatment of cancer in China, but the material basis and potential mechanisms are still limited. The present study aimed to use in vitro experiments, UPLC-Q-TOF/MS and network pharmacology to predict active compounds and possible mechanisms of cultivated and wild I. hispidus. The cytotoxicity results in vitro showed that the extracts of cultivated and wild fruit bodies exhibited the highest inhibitory effects against MDA-MB-231 cells, and the 50% inhibition concentration, (IC50) values were 59.82 and 92.09 mug/mL, respectively. Of the two extracts, a total of 30 possible chemical components, including 21 polyphenols and nine fatty acids, were identified. Network pharmacology showed that five active polyphenols (osmundacetone, isohispidin, inotilone, Hispolon, and inonotusin A) and 11 potential targets (HSP90AA1, AKT1, STAT3, EGFR, ESR1, PIK3CA, HIF1A, ERBB2, TERT, EP300 and HSP90AB1) were found to be closely associated with antitumor activity. Furthermore, 18 antitumor-related pathways were identified using the compound-target-pathway network. The molecular docking revealed that the active polyphenols had a good binding ability to the core targets, and the results were consistent with those of network pharmacology. Based on these findings, we speculate that I. hispidus can exert its antitumor activity through multicomponent, multitarget, and multichannel mechanisms of action.
Protective role of hispolon and its derivatives against apoptosis in cortical neurons induced by electromagnetic radiation from 4G mobile phone.[Pubmed:36994543]
J Biochem Mol Toxicol. 2023 Jul;37(7):e23351.
Electromagnetic radiation (EMR) from wireless devices, particularly mobile phones, is a potentially growing public health concern. In this study, the neuronal effects of EMR on primary cortical neurons (PCNs) from neonatal rat cerebral cortex and the protective role of Hispolon (HIS) and its derivatives were investigated as a measure of cranial exposure during mobile phone use. PCNs were isolated and cultured from day-old neonatal rats, then exposed for 2 h to EMR emitted by a mobile phone operating at a frequency of 2100 MHz with 1.6 W/Kg specific absorption rate (SAR) in call-answered mode treated with HIS and its derivatives. The induction of apoptosis through modulation of pro and anti-apoptotic genes via mitochondrial pathway and the protection by the test compounds was assessed. Pyrazole derivatives decreased apoptosis by modulating the levels of pro and anti-apoptotic genes by reducing the levels of reactive oxygen species (ROS) via mitochondrial damage, which was observed in the EMR exposed PCNs. The pyrazole compounds were found to have antioxidative and anti-apoptotic properties. Thus, the neuroprotective mechanisms of the pyrazole derivatives can be investigated further, which may make them appropriate as lead compounds in developing neuroprotective formulations.
Hispolon induces apoptosis in oral squamous cell carcinoma cells through JNK/HO-1 pathway activation.[Pubmed:36967712]
J Cell Mol Med. 2023 May;27(9):1250-1260.
Oral squamous cell carcinoma (OSCC) has a high recurrence rate and poor prognosis. Hispolon, a polyphenolic compound with antiviral, antioxidant, and anticancer activities, is a potential chemotherapy agent. However, few studies have investigated the anti-cancer mechanism of Hispolon in oral cancer. This present study used the cell viability assay, clonogenic assay, fluorescent nuclear staining, and flow cytometry assay to analyse the apoptosis-inducing effects of Hispolon in OSCC cells. After Hispolon treatment, the apoptotic initiators, cleaved caspase-3, -8, and - 9, were upregulated, whereas the cellular inhibitor of apoptosis protein-1 (cIAP1) was downregulated. Furthermore, a proteome profile analysis using a human apoptosis array revealed the overexpression of heme oxygenase-1 (HO-1) by Hispolon, which was determined to be involved in caspase-dependent apoptosis. Moreover, cotreatment with Hispolon and mitogen-activated protein kinase (MAPK) inhibitors revealed that Hispolon induces apoptosis in OSCC cells through activation of the c-Jun N-terminal kinase (JNK) pathway and not the extracellular signal-regulated kinase (ERK) or p38 pathway. These findings indicate that Hispolon may exert an anticancer effect on oral cancer cells by upregulating HO-1 and inducing caspase-dependent apoptosis by activating the JNK pathway.
Hispolon Methyl Ether, a Hispolon Analog, Suppresses the SRC/STAT3/Survivin Signaling Axis to Induce Cytotoxicity in Human Urinary Bladder Transitional Carcinoma Cell Lines.[Pubmed:36613579]
Int J Mol Sci. 2022 Dec 21;24(1):138.
Bladder cancer is a leading human malignancy worldwide. Signal transducer and activator of transcription (STAT) 3 is an oncogenic transcription factor commonly hyperactivated in most human cancers, including bladder cancer. Notably, preclinical evidence has validated STAT3 blockade as a promising therapeutic strategy for bladder cancer. Hispolon Methyl Ether (HME) is a structural analog of Hispolon, an anticancer component of the medicinal mushroom Phellinus linteus. Thus far, HME's anticancer activity and mechanisms remain largely unknown. We herein report HME was cytotoxic, more potent than cisplatin, and proapoptotic to various human bladder transitional carcinoma cell lines. Of note, HME blocked STAT3 activation, evidenced by HME-elicited reduction in tyrosine 705-phosphorylated STAT3 levels constitutively expressed or induced by interleukin-6. Significantly, HME-induced cytotoxicity was abrogated in cells expressing a dominant-active STAT3 mutant (STAT3-C), confirming STAT3 blockage as a pivotal mechanism of HME's cytotoxic action. We further revealed that survivin was downregulated by HME, while its levels were rescued in STAT3-C-expressing cells. Moreover, survivin overexpression abolished HME-induced cytotoxicity, illustrating survivin as a central downstream mediator of STAT3 targeted by HME. Lastly, HME was shown to lower tyrosine 416-phosphorylated SRC levels, suggesting that HME inhibits STAT3 by repressing the activation of SRC, a STAT3 upstream kinase. In conclusion, we present the first evidence of HME's anti-bladder cancer effect, likely proceeding by evoking apoptosis through suppression of the antiapoptotic SRC/STAT3/survivin signaling axis.
Hispolon Cyclodextrin Complexes and Their Inclusion in Liposomes for Enhanced Delivery in Melanoma Cell Lines.[Pubmed:36430965]
Int J Mol Sci. 2022 Nov 21;23(22):14487.
Hispolon, a phenolic pigment isolated from the mushroom species Phellinus linteus, has been investigated for anti-inflammatory, antioxidant, and anticancer properties; however, low solubility and poor bioavailability have limited its potential clinical translation. In this study, the inclusion complex of Hispolon with Sulfobutylether-beta-cyclodextrin (SBEbetaCD) was characterized, and the Hispolon-SBEbetaCD Complex (HSC) was included within the sterically stabilized liposomes (SL) to further investigate its anticancer activity against melanoma cell lines. The HSC-trapped-Liposome (HSC-SL) formulation was investigated for its sustained drug delivery and enhanced cytotoxicity. The inclusion complex in the solid=state was confirmed by a Job's plot analysis, molecular modeling, differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), Proton nuclear magnetic resonance (NMR) spectroscopy, and scanning electron microscopy (SEM). The HSC-SL showed no appreciable deviation in size (<150 nm) and polydispersity index (<0.2) and improved drug encapsulation efficiency (>90%) as compared to control Hispolon liposomes. Individually incorporated Hispolon and SBEbetaCD in the liposomes (H-CD-SL) was not significant in loading the drug in the liposomes, compared to HSC-SL, as a substantial amount of free drug was separated during dialysis. The HSC-SL formulation showed a sustained release compared to Hispolon liposomes (H-SLs) and Hispolon-SBEbetaCD liposomes (H-CD-SLs). The anticancer activity on melanoma cell lines (B16BL6) of HSC and HSC-SL was higher than in H-CD-SL and Hispolon solution. These findings suggest that HSC inclusion in the HSC-SL liposomes stands out as a potential formulation approach for enhancing drug loading, encapsulation, and chemotherapeutic efficiency of Hispolon and similar water insoluble drug molecules.
Immunomodulatory Effect of Hispolon on LPS-Induced RAW264.7 Cells and Mitogen/Alloantigen-Stimulated Spleen Lymphocytes of Mice.[Pubmed:35890318]
Pharmaceutics. 2022 Jul 6;14(7):1423.
Hispolon is a potent anticancer, anti-inflammatory, antioxidant, and antidiabetic agent isolated from Phellinus linteus, an oriental medicinal mushroom. However, the immunomodulatory mechanisms by which Hispolon affects macrophages and lymphocytes remain poorly characterized. We investigated the immunomodulatory effects of Hispolon on oxidative stress, inflammatory responses, and lymphocyte proliferation using lipopolysaccharide (LPS)-treated RAW264.7 macrophages or mitogen/alloantigen-treated mouse splenocytes. Hispolon inhibited LPS-induced reactive oxygen and nitrogen species (ROS/RNS) generation and decreased total sulfhydryl (SH) levels in a cell-free system and RAW264.7 cells. Hispolon exerted significant anti-inflammatory effects by inhibiting production of the proinflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) and activation of nuclear factor kappa B (NF-kappaB) and signal transducer and activator of transcription 3 (STAT3) in LPS-treated RAW264.7 cells. Hispolon also modulated NF-kappaB and STAT3 activation by suppressing the NF-kappaB p65 interaction with phospho-IkappaBalpha and the STAT3 interaction with JAK1, as determined via coimmunoprecipitation analysis. Additionally, Hispolon significantly decreased lymphocyte proliferation, T cell responses and T helper type 1 (Th1)/type 2 (Th2) cytokines production in mitogen/alloantigen-treated splenocytes. We conclude that Hispolon exerts immunomodulatory effects on LPS-treated macrophages or mitogen/alloantigen-treated splenocytes through antioxidant, anti-inflammatory, and antiproliferative activities. Thus, Hispolon may be a therapeutic agent for treating immune-mediated inflammatory diseases.
Natural Compounds or Their Derivatives against Breast Cancer: A Computational Study.[Pubmed:35837379]
Biomed Res Int. 2022 Jul 5;2022:5886269.
BACKGROUND: Breast cancer is one of the most common types of cancer diagnosed and the second leading cause of death among women. Breast cancer susceptibility proteins of type 1 and 2 are human tumor suppressor genes. Genetic variations/mutations in these two genes lead to overexpression of human breast tumor suppressor genes (e.g., BRCA1, BRCA2), which triggers uncontrolled duplication of cells in humans. In addition, multidrug resistance protein 1 (MDR1), an important cell membrane protein that pumps many foreign substances from cells, is also responsible for developing resistance to cancer chemotherapy. Aim of the Study. The aim of this study was to analyze some natural compounds or their derivatives as part of the development of strong inhibitors for breast cancer. Methodology. Molecular docking studies were performed using compounds known in the literature to be effective against BRCA1 and BRCA2 and MDR1, with positive control being 5-fluorouracil, an antineoplastic drug as a positive control. RESULTS: The binding affinity of the compounds was analyzed, and it was observed that they had a better binding affinity for the target proteins than the standard drug 5-fluorouracil. Among the compounds analyzed, alpha-hederin, andrographolide, apigenin, asiatic acid, auricular acid, sinularin, curcumin, citrinin, Hispolon, nerol, phytol, retinol palmitate, and sclareol showed the best binding affinity energy to the BRCA1, BRCA2, and MDR1 proteins, respectively. CONCLUSIONS: alpha-Hederin, andrographolide, apigenin, asiatic acid, auricular acid, Hispolon, sclareol, curcumin, citrinin, and sinularin or their derivatives can be a good source of anticancer agents in breast cancer.
Hispolon alleviates oxidative damage by stimulating the Nrf2 signaling pathway in PC12 cells.[Pubmed:35660410]
Arch Biochem Biophys. 2022 Sep 30;727:109303.
Natural products derived from the daily diet are garnering increasing attention for neurodegenerative disease (ND) treatment. Hispolon (His), a small molecule from Phellinus linteus, has been reported to have various pharmacological activities. Here, we evaluated its protective effect on a neuron-like rat pheochromocytoma cell line (PC12). Results showed that His could restore cell death induced by oxidative damage. Nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2) plays a significant role in maintaining cellular redox homeostasis. After treatment with His, some Nrf2-governed antioxidant genes were upregulated in a dose-dependent manner. However, the protective effect of His on PC12 cells was easily terminated by Nrf2 knockdown, demonstrating that Nrf2 is a critical component in this cytoprotective process. Taken together, our study showed that His was not only an effective activator of Nrf2 but also a promising candidate for ND treatment.
Hispolon-Loaded Liquid Crystalline Nanoparticles: Development, Stability, In Vitro Delivery Profile, and Assessment of Hepatoprotective Activity in Hepatocellular Carcinoma.[Pubmed:35350323]
ACS Omega. 2022 Mar 10;7(11):9452-9464.
The present work describes the development and characterization of liquid crystalline nanoparticles of Hispolon (HP-LCNPs) for treating hepatocellular carcinoma. HP-LCNPs were prepared by a top-down method utilizing GMO as the lipid and Pluronic F-127 as the polymeric stabilizer. The prepared formulations (HP1-HP8) were tested for long-term stability, where HP5 showed good stability with a particle size of 172.5 +/- 0.3 nm, a polydispersity index (PDI) of 0.38 +/- 0.31 nm, a zeta potential of -10.12 mV +/- 0.05, an entrapment efficiency of 86.81 +/- 2.5%, and a drug loading capacity of 12.51 +/- 1.12%. Optical photomicrography and transmission electron microscopy images demonstrated a consistent, low degree of aggregation and a spherical shape of LCNPs. The effect of temperature and pH on the optimized formulation (HP5) indicated good stability at 45 degrees C and at pH between 2 and 5. In vitro gastrointestinal stability indicated no significant change in the particle size, PDI, and entrapment efficiency of the drug. The drug release study exhibited a biphasic pattern in simulated gastric fluid (pH 1.2) for 2 h and simulated intestinal fluid (pH 7.4) for up to 24 h, while the best fitting of the profile was observed with the Higuchi model, indicating the Fickian diffusion mechanism. The in vivo pharmacokinetic study demonstrated nearly 4.8-fold higher bioavailability from HP5 (AUC: 1774.3 +/- 0.41 mug* h/mL) than from the HP suspension (AUC: 369.11 +/- 0.11 mug* h/mL). The anticancer activity evaluation revealed a significant improvement in antioxidant parameters and serum hepatic biomarkers (SGOT, SGPT, ALP, total bilirubin, and GGT) in the diethyl nitrosamine-treated group of rats with the optimized LCNP formulation (HP5) vis-a-vis HP suspension.
Curcumin analogues with improved antioxidant properties: A theoretical exploration.[Pubmed:34763936]
Food Chem. 2022 Mar 30;373(Pt B):131499.
Curcumin, a ubiquitous dietary molecule, is a versatile antioxidant that fights against free radicals. The antioxidant activity of curcumin and its structural analogues such as Hispolon, halfcurcumin and polyhydroxycurcumin is analyzed using density functional theory (DFT). The thermochemical parameter, bond dissociation enthalpy (BDE) is used to analyse the propensity of radical attack. The hydrogen atom transfer (HAT) energetics for the hydroxyl groups of the antioxidant molecules with *OH and *OOH in both gas and solvent media are explored. Based on ourresults, Hispolon and polyhydroxycurcumin characterized by multiple hydroxyl groups arranged in ortho dihydroxy fashion are good radical scavengers. Halfcurcumin exhibited comparatively similar activity as that of curcumin. The absorption properties of these molecules are in good agreement with the reported experimental findings. The molecular docking studies revealed that these antioxidants can indirectly control the oxidative stress by favourably interacting with the pro-oxidant enzyme like xanthine oxidase.


