Gomisin L2CAS# 82425-44-3 |
Quality Control & MSDS
Number of papers citing our products

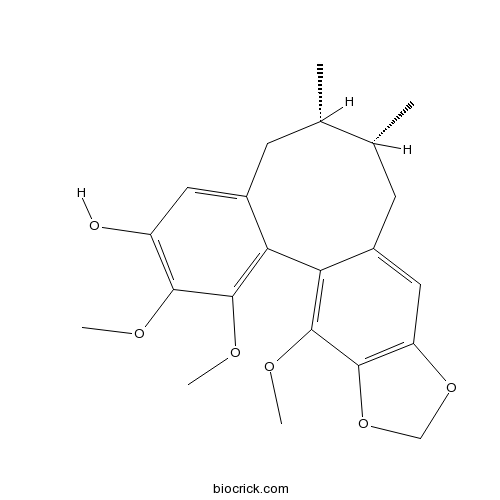
Chemical structure

3D structure
| Cas No. | 82425-44-3 | SDF | Download SDF |
| PubChem ID | 5317807 | Appearance | Powder |
| Formula | C22H26O6 | M.Wt | 386.44 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (9S,10R)-3,4,19-trimethoxy-9,10-dimethyl-15,17-dioxatetracyclo[10.7.0.02,7.014,18]nonadeca-1(19),2,4,6,12,14(18)-hexaen-5-ol | ||
| SMILES | CC1CC2=CC(=C(C(=C2C3=C(C4=C(C=C3CC1C)OCO4)OC)OC)OC)O | ||
| Standard InChIKey | BVMLGLOHSDNEJG-NWDGAFQWSA-N | ||
| Standard InChI | InChI=1S/C22H26O6/c1-11-6-13-8-15(23)19(24-3)21(25-4)17(13)18-14(7-12(11)2)9-16-20(22(18)26-5)28-10-27-16/h8-9,11-12,23H,6-7,10H2,1-5H3/t11-,12+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gomisin L2 is a natural product from Schizandra chinensis BAILL. |
| Structure Identification | Chemical & Pharmaceutical Bulletin, 1982, 30(1):132-139.The Constituents of Schizandra chinensis Baill. X. The Structures of .GAMMA.-schizandrin and four new lignans,(-)-gomisins L1 and L2,(.+-.)-gomisin M1 and (+)-gomisin M2.[Reference: WebLink]
|

Gomisin L2 Dilution Calculator

Gomisin L2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5877 mL | 12.9386 mL | 25.8772 mL | 51.7545 mL | 64.6931 mL |
| 5 mM | 0.5175 mL | 2.5877 mL | 5.1754 mL | 10.3509 mL | 12.9386 mL |
| 10 mM | 0.2588 mL | 1.2939 mL | 2.5877 mL | 5.1754 mL | 6.4693 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5175 mL | 1.0351 mL | 1.2939 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5175 mL | 0.6469 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gomisin L1
Catalog No.:BCN7039
CAS No.:82425-43-2
- 20-O-Acetylingenol-3-angelate
Catalog No.:BCN8469
CAS No.:82425-35-2
- Ofloxacin
Catalog No.:BCC2526
CAS No.:82419-36-1
- Ganciclovir
Catalog No.:BCC4908
CAS No.:82410-32-0
- Humantenirine
Catalog No.:BCN4850
CAS No.:82375-30-2
- Humantenine
Catalog No.:BCN4358
CAS No.:82375-29-9
- Heliocurassavinine
Catalog No.:BCN2050
CAS No.:82374-02-5
- 2,3,5,4'-Tetrahydroxyl diphenylethylene-2-O-glucoside
Catalog No.:BCN1340
CAS No.:82373-94-2
- Humantenmine
Catalog No.:BCN4357
CAS No.:82354-38-9
- Heliocurassavicine
Catalog No.:BCN2049
CAS No.:82354-34-5
- Heliocoromandaline
Catalog No.:BCN2046
CAS No.:82354-33-4
- Coelonin
Catalog No.:BCN3600
CAS No.:82344-82-9
- Gomisin M2
Catalog No.:BCN4359
CAS No.:82425-45-4
- Maglifloenone
Catalog No.:BCN4360
CAS No.:82427-77-8
- Agomelatine L(+)-Tartaric acid
Catalog No.:BCC4211
CAS No.:824393-18-2
- Pulchinenoside E3
Catalog No.:BCN8187
CAS No.:824401-07-2
- Arteannuin A
Catalog No.:BCN4361
CAS No.:82442-48-6
- Hispanone
Catalog No.:BCN7404
CAS No.:82462-67-7
- 3-Oxotirucalla-7,24-dien-21-oic acid
Catalog No.:BCN4568
CAS No.:82464-35-5
- R(+)-Gomisin M1
Catalog No.:BCN4362
CAS No.:82467-50-3
- Neokadsuranin
Catalog No.:BCN7816
CAS No.:115181-68-5
- Oroxylin A 7-O-beta-D-glucuronide methyl ester
Catalog No.:BCN1339
CAS No.:82475-01-2
- Baicalin methyl ester
Catalog No.:BCN3252
CAS No.:82475-03-4
- LP 44
Catalog No.:BCC7399
CAS No.:824958-12-5
Kudsuphilactone B, a nortriterpenoid isolated from Schisandra chinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer A2780 cells.[Pubmed:28229391]
Arch Pharm Res. 2017 Apr;40(4):500-508.
A phytochemical study on the fruits of Schisandra chinensis led to the isolation and characterization of nineteen compounds. The structures of the isolates were determined to be schizandrin, deoxyschizandrin, angeloylgomisin H, gomisin A, gomisin J, (-)-gomisin L1, (-)-Gomisin L2, wuweizisu C, gomisin N, meso-dihydroguaiaretic acid, kadsuphilactone B, alpha-ylangenol, alpha-ylangenyl acetate, beta-chamigrenal, beta-chamigrenic acid, 4-hydroxybenzoic acid, protocatechuic acid, p-methylcarvacrol, and indole-3-acetic acid. Of these, some lignans and a nortriterpene showed cytotoxic activity in human ovarian and endometrial cancer cells. In particular, a nortriterpenoid kadsuphilactone B exhibited significant cytotoxic activity with IC50 values below 25 muM in both A2780 and Ishikawa cells. Kadsuphilactone B induced apoptotic cell death and stimulated the activation of caspase-3, -8, and -9 and the cleavages of poly (ADP-ribose) polymerase. Caspase inhibitors attenuated the pro-apoptotic activity of kudsuphilactone B. In addition, kadsuphilactone B altered the expression levels of B cell lymphoma 2 (Bcl-2) family proteins. Moreover, activation of MAPKs was modulated by kadsuphilactone B in a dose-dependent manner. Taken together, these results show that kadsuphilactone B induces caspase-dependent apoptosis in human cancer cells via the regulation of Bcl-2 family protein and MAPK signaling.


