GirinimbineCAS# 23095-44-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23095-44-5 | SDF | Download SDF |
| PubChem ID | 96943 | Appearance | Powder |
| Formula | C18H17NO | M.Wt | 263.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
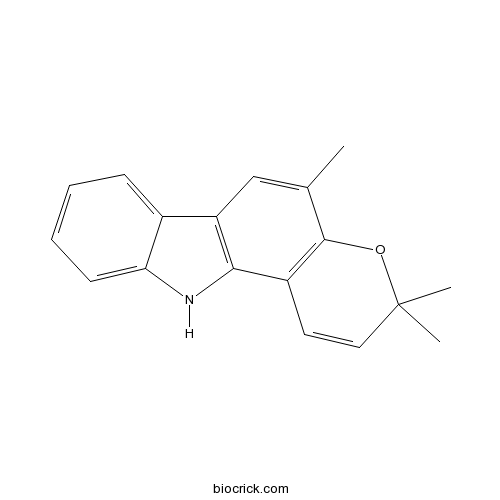
| Chemical Name | 3,3,5-trimethyl-11H-pyrano[3,2-a]carbazole | ||
| SMILES | CC1=CC2=C(C3=C1OC(C=C3)(C)C)NC4=CC=CC=C42 | ||
| Standard InChIKey | GAEQWKVGMHUUKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H17NO/c1-11-10-14-12-6-4-5-7-15(12)19-16(14)13-8-9-18(2,3)20-17(11)13/h4-10,19H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Girinimbine Dilution Calculator

Girinimbine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7979 mL | 18.9897 mL | 37.9795 mL | 75.959 mL | 94.9487 mL |
| 5 mM | 0.7596 mL | 3.7979 mL | 7.5959 mL | 15.1918 mL | 18.9897 mL |
| 10 mM | 0.3798 mL | 1.899 mL | 3.7979 mL | 7.5959 mL | 9.4949 mL |
| 50 mM | 0.076 mL | 0.3798 mL | 0.7596 mL | 1.5192 mL | 1.899 mL |
| 100 mM | 0.038 mL | 0.1899 mL | 0.3798 mL | 0.7596 mL | 0.9495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Visartiside E
Catalog No.:BCN9349
CAS No.:1212005-08-7
- 8-Hydroxythymol
Catalog No.:BCN9348
CAS No.:4478-33-5
- 2α,3α,24-Trihydroxyursa-12,20(30)-dien-28-oic acid
Catalog No.:BCN9347
CAS No.:341503-22-8
- Phochinenin I
Catalog No.:BCN9346
CAS No.:1070883-77-0
- Murrayafoline A
Catalog No.:BCN9345
CAS No.:4532-33-6
- Thymyl 2-methylbutyrate
Catalog No.:BCN9344
CAS No.:69844-32-2
- Methyl ganoderenate D
Catalog No.:BCN9343
CAS No.:748136-03-0
- Methyl lucidenate N
Catalog No.:BCN9342
CAS No.:1276655-49-2
- Cadambine
Catalog No.:BCN9341
CAS No.:54422-49-0
- 8,9-Dehydro-7,9-diisobutyryloxythymol
Catalog No.:BCN9340
CAS No.:22518-03-2
- Hancolupenone
Catalog No.:BCN9339
CAS No.:132746-04-4
- Phochinenin G
Catalog No.:BCN9338
CAS No.:1070883-75-8
- Benzyl [5-O-benzoyl-β-D-apiofuranosyl(1→2)]-β-D-glucopyranoside
Catalog No.:BCN9351
CAS No.:1097040-08-8
- Pomegralignan
Catalog No.:BCN9352
CAS No.:1562492-32-3
- Methyl lucidenate D
Catalog No.:BCN9353
CAS No.:98665-09-9
- 1-[2-Hydroxy-4-(hydroxymethyl)phenyl]ethanone
Catalog No.:BCN9354
CAS No.:22518-00-9
- Methyl lucidenate A
Catalog No.:BCN9355
CAS No.:105742-79-8
- 3-Hydroxyperillaldehyde
Catalog No.:BCN9356
CAS No.:1932348-11-2
- Convallagenin B
Catalog No.:BCN9357
CAS No.:17934-59-7
- Juncusol 2-O-glucoside
Catalog No.:BCN9358
CAS No.:175094-14-1
- Betulinic acid palmitate
Catalog No.:BCN9359
CAS No.:19833-15-9
- 6,6'-Di-O-sinapoylsucrose
Catalog No.:BCN9360
CAS No.:1068661-35-7
- Ethyl (9Z,11E)-13-hydroxyoctadeca-9,11-dienoate
Catalog No.:BCN9361
CAS No.:947529-92-2
- Ethyl brevifolincarboxylate
Catalog No.:BCN9362
CAS No.:107646-82-2
Girinimbine from curry leaves promotes gastro protection against ethanol induced peptic ulcers and improves healing via regulation of anti-inflammatory and antioxidant mechanisms.[Pubmed:32248216]
Food Funct. 2020 Apr 30;11(4):3493-3505.
Curry leaves (Murraya koenigii) are a leafy spice used in Indian cookery for its fragrant aroma. Many bioactive functional compounds have been identified, and among them carbazole alkaloids have attracted wide attention due to their multi-dimensional medicinal value. Even though it has been established that the carbazole alkaloid is responsible for the anti-ulcer effect showed by this culinary herb, there is no further evidence to say which phytochemical is responsible for this. In the present study, we investigated the gastro-protective effects and mechanism of Girinimbine, a major carbazole alkaloid present in curry leaves. Rats were administered with ethanol to produce gastric ulcers, and the prophylactic effect of Girinimbine was evaluated. A macroscopic and histological examination was carried out to examine the lesions. Furthermore, the mucus production, NO production, PGE2 synthesis, mucosal nonprotein sulphydryls, glutathione (GSH) level, lipid peroxidation (MDA) level and COX inhibition were assessed. In addition, in particular, TNF-alpha and IL-6, two important cytokines, were evaluated. Immunohistochemical and gene expression studies were conducted to determine the HSP70 and iNOS biomarkers. Our results indicated that Girinimbine significantly reduced the ulcer index and totally safeguarded the mucosa from lesions. The protective effect of Girinimbine was complemented through the restoration of the reduced GSH and NP-SH level. This was associated with a reduction of MDA, which was elevated by the administration of ethanol. Pre-treatment of the ethanol induced ulcer with Girinimbine reduced the NO concentration in the plasma and elevated PGE2 together with a decreased level of TNF-alpha and IL-6. Girinimbine had shown suppressing effects on COX-2 enzymes, but not on COX-1. In addition, significantly upregulated HSP70 and downregulated iNOS were observed in Girinimbine treated rat tissue at both the transcriptional and translational level. Our results clearly indicated that Girinimbine displayed a significant gastro-protection effect, via the capacity to inhibit inflammatory responses and antioxidant potential.
[Chemical constituents from stems and leaves of Clausena emarginata].[Pubmed:31355567]
Zhongguo Zhong Yao Za Zhi. 2019 May;44(10):2096-2101.
The chemical constituents from the stems and leaves of Clausena emarginata were separated and purified by column chromatographies on silica gel,ODS,Sephadex LH-20,and PR-HPLC. The structures of the isolated compounds were identified on the basis of physicochemical properties and spectroscopic analysis,as well as comparisons with the data reported in the literature. Sixteen compounds were isolated from the 90% ethanol extract of the stems and leaves of C. emarginata,which were identified as siamenol( 1),murrastanine A( 2),3-formyl-1,6-dimethoxycarbazole( 3),3-methoxymethylcarbazole( 4),3-methylcarbazole( 5),murrayafoline A( 6),3-formylcarbazole( 7),3-formyl-1-hydroxycarbazole( 8),3-formyl-6-methoxycarbazole( 9),murrayanine( 10),murrayacine( 11),Girinimbine( 12),nordentatin( 13),chalepin( 14),8-hydroxy-6-methoxy-3-pentylisocoumarin( 15) and ethyl orsellinate( 16). Compounds 1-4,14-16 were isolated from C. emarginata for the first time. Among them,compounds 1,2,15 and 16 were isolated from the genus Clausena for the first time. All isolated compounds were evaluated for their cytotoxic activities against five human cancer cell lines: HL-60,SMMC-7721,A-549,MCF-7 and SW480 in vitro. Compounds 12 and 14 showed significant inhibitory effects against various human cancer cell lines with IC_(50) values comparable to those of doxorubicin.
Pleiotropic Effect of Mahanine and Girinimbine Analogs: Anticancer Mechanism and its Therapeutic Versatility.[Pubmed:30173653]
Anticancer Agents Med Chem. 2018;18(14):1983-1990.
Emerging evidence present credible support in favour of the potential role of mahanine and Girinimbine. Non-toxic herbal carbazole alkaloids occur in the edible part of Murraya koenigii, Micromelum minutum, M. zeylanicum, and M. euchrestiolia. Mahanine and Girinimbine are the major potent compounds from these species. In fact, they interfered with tumour expansion and metastasis development through down-regulation of apoptotic and antiapoptotic protein, also involved in the stimulation of cell cycle arrest. Consequently, these compounds were well proven for the in-vitro and in vivo evaluation that could be developed as novel agents either alone or as an adjuvant to conventional therapeutics. Therefore, mahanine and Girinimbine analogs have the potential to be the promising chemopreventive agents for the tumour recurrence and the treatment of human malignancies. In this review, an updated wide-range of pleiotropic anticancer and biological effects induction by mahanine and Girinimbine against cancer cells were deeply summarized.
Girinimbine Inhibits the Proliferation of Human Ovarian Cancer Cells In Vitro via the Phosphatidylinositol-3-Kinase (PI3K)/Akt and the Mammalian Target of Rapamycin (mTOR) and Wnt/beta-Catenin Signaling Pathways.[Pubmed:30084434]
Med Sci Monit. 2018 Aug 7;24:5480-5487.
BACKGROUND Worldwide, ovarian cancer is increasing in prevalence and has a high mortality rate. Girinimbine is a carbazole alkaloid isolated from Murraya koenigii (the curry tree) and is used in Chinese herbal medicine. The aim of this study was to evaluate the effects of Girinimbine on cell proliferation, cell migration, and apoptosis in human ovarian cancer cells in vitro. MATERIAL AND METHODS A human ovarian cancer cell line panel, which included SKOV3 cells and the SV40 immortalized normal human ovarian cell line, were treated with increasing doses of Girinimbine. Cell proliferation was evaluated using the MTT assay. Confocal immunofluorescence using 4',6-diamidino-2-phenylindole (DAPI), Annexin-V, and propidium iodide (PI) were used to measure cell apoptosis. Cell migration and invasion were determined by transwell assays. Protein expression was determined by Western blot. RESULTS Girinimbine inhibited SKOV3 ovarian cancer cell proliferation in a dose-dependent manner. The half maximal inhibitory concentration (IC50) of grinimbine was 15 microM for the SKOV3 cells, and 120 microM for the SV40 cells. Grinimbine treatment resulted in apoptosis of SKOV3 cells, from 2.2% in untreated cells to 58.8% at a dose of 30 microM, which was associated with an increase in the Bax/Bcl-2 ratio. Girinimbine inhibited cell migration and invasion of the SKOV3 cancer cells in vitro and inhibited the PI3K/AKT/mTOR and Wnt/beta-catenin signaling pathways. CONCLUSIONS Girinimbine, a carbazole alkaloid used in Chinese herbal medicine, inhibited the proliferation and cell migration of human ovarian cancer cells in vitro, in a dose-dependent manner, via the PI3K/Akt/mTOR and Wnt/beta-catenin signaling pathways.
Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii.[Pubmed:28096658]
Drug Des Devel Ther. 2016 Dec 28;11:103-121.
Therapy that directly targets apoptosis and/or inflammation could be highly effective for the treatment of cancer. Murraya koenigii is an edible herb that has been traditionally used for cancer treatment as well as inflammation. Here, we describe that Girinimbine, a carbazole alkaloid isolated from M. koenigii, induced apoptosis and inhibited inflammation in vitro as well as in vivo. Induction of apoptosis in human colon cancer cells (HT-29) by Girinimbine revealed decreased cell viability in HT-29, whereas there was no cytotoxic effect on normal colon cells. Changes in mitochondrial membrane potential, nuclear condensation, cell permeability, and cytochrome c translocation in Girinimbine-treated HT-29 cells demonstrated involvement of mitochondria in apoptosis. Early-phase apoptosis was shown in both acridine orange/propidium iodide and annexin V results. Girinimbine treatment also resulted in an induction of G0/G1 phase arrest which was further corroborated with the upregulation of two cyclin-dependent kinase proteins, p21 and p27. Girinimbine treatment activated apoptosis through the intrinsic pathway by activation of caspases 3 and 9 as well as cleaved caspases 3 and 9 which ended by triggering the execution pathway. Moreover, apoptosis was confirmed by downregulation of Bcl-2 and upregulation of Bax in Girinimbine-treated cells. In addition, the key tumor suppressor protein, p53, was seen to be considerably upregulated upon Girinimbine treatment. Induction of apoptosis by Girinimbine was also evidenced in vivo in zebrafish embryos, with results demonstrating significant distribution of apoptotic cells in embryos after a 24-hour treatment period. Meanwhile, anti-inflammatory action was evidenced by the significant dose-dependent Girinimbine inhibition of nitric oxide production in lipopolysaccharide/interferon-gamma-induced cells along with significant inhibition of nuclear factor-kappa B translocation from the cytoplasm to nucleus in stimulated RAW 264.7 cells. Girinimbine was also shown to have considerable antioxidant activity whereby 20 mug/mL of Girinimbine was equivalent to 82.17+/-1.88 muM of Trolox. In mice with carrageenan-induced peritonitis, oral pretreatment with Girinimbine helped limit total leukocyte migration (mainly of neutrophils), and reduced pro-inflammatory cytokine levels (interleukin-1beta and tumor necrosis factor-alpha) in the peritoneal fluid. These findings strongly suggest that Girinimbine could act as a chemopreventive and/or chemotherapeutic agent by inducing apoptosis while suppressing inflammation. There is a potential for Girinimbine to be further investigated for its applicability in treating early stages of cancer.
Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities.[Pubmed:26947457]
Org Biomol Chem. 2016 Mar 28;14(12):3322-32.
In our present study, four new, designated as murrayakonine A-D (), along with 18 known carbazole alkaloids were isolated from CHCl3 : MeOH (1 : 1) crude extracts of the stems and leaves of Murraya koenigii (Linn.) Spreng. The structures of the all isolated compounds were characterized by analysis of HR-ESI-MS and NMR (1D and 2D spectroscopy) results, and comparison of their data with the literature data. For the first time, all the isolates were evaluated for their anti-inflammatory activities, using both in vitro and in vivo experiments, against the key inflammatory mediators TNF-alpha and IL-6. The new compound murrayakonine A (), O-methylmurrayamine A () and murrayanine () were proven to be the most active, efficiently inhibiting TNF-alpha and IL-6 release in a dose-dependent manner and showing decreased LPS induced TNF-alpha and IL-6 production in human PBMCs [corrected]. Furthermore, all the isolates were screened for their antimicrobial potential, and the compounds Girinimbine () (IC50 3.4 muM) and 1-hydroxy-7-methoxy-8-(3-methylbut-2-en-1-yl)-9H-carbazole-3-carbaldehyde () (IC50 10.9 muM) displayed potent inhibitory effects against Bacillus cereus. Furthermore, compounds murrayamine J () (IC50 11.7 muM) and koenimbine () (IC50 17.0 muM) were active against Staphylococcus aureus. However, none of the compounds were found to be active against Escherichia coli or Candida albicans.
In vitro and in vivo anti-angiogenic activity of girinimbine isolated from Murraya koenigii.[Pubmed:25767375]
Drug Des Devel Ther. 2015 Mar 3;9:1281-92.
Girinimbine is a carbazole alkaloid isolated from the stem bark and root of Murraya koenigii. Here we report that Girinimbine is an inhibitor of angiogenic activity both in vitro and in vivo. MTT results showed that Girinimbine inhibited proliferation of human umbilical vein endothelial cells, while results from endothelial cell invasion, migration, tube formation, and wound healing assays demonstrated significant time- and dose-dependent inhibition by Girinimbine. A proteome profiler array done on Girinimbine-treated human umbilical vein endothelial cells showed that Girinimbine had mediated regulation of pro-angiogenic and anti-angiogenic proteins. The anti-angiogenic potential of Girinimbine was also evidenced in vivo in the zebrafish embryo model wherein Girinimbine inhibited neo vessel formation in zebrafish embryos following 24 hours of exposure. Together, these results showed that Girinimbine could effectively suppress angiogenesis, suggestive of its therapeutic potential as a novel angiogenesis inhibitor.
Efficient construction of pyrano[3,2-a]carbazoles: application to a biomimetic total synthesis of cyclized monoterpenoid pyrano[3,2-a]carbazole alkaloids.[Pubmed:24030919]
Chemistry. 2013 Oct 11;19(42):14098-111.
We have developed a highly efficient route to 2-hydroxy-3-methylcarbazole (1) via a palladium-catalyzed construction of the carbazole skeleton. Using 1 as relay compound, different methods for annulations of pyran rings by reaction with terpenoid building blocks have been tested. The Lewis acid promoted reaction of 1 with prenal (21) opened up an efficient route to Girinimbine (3) and the corresponding reaction with citral (25) afforded mahanimbine (5). Oxidation of compounds 3 and 5 provided murrayacine (4) and murrayacinine (6). Following the biogenetic proposal, mahanimbine (5) has been exploited for efficient biomimetic syntheses of the cyclized monoterpenoid pyrano[3,2-a]carbazole alkaloids cyclomahanimbine (7), mahanimbidine (8) and bicyclomahanimbine (9). The interconversions of 5, 7, 8 and 9 are described and mechanistic implications are discussed. Structural assignments are unambiguously verified by X-ray crystal structure determinations. Moreover, cyclomahanimbine (7) was transformed into murrayazolinine (10) and exozoline (11).
Apoptosis Effect of Girinimbine Isolated from Murraya koenigii on Lung Cancer Cells In Vitro.[Pubmed:23573145]
Evid Based Complement Alternat Med. 2013;2013:689865.
Murraya koenigii Spreng has been traditionally claimed as a remedy for cancer. The current study investigated the anticancer effects of Girinimbine, a carbazole alkaloid isolated from Murraya koenigii Spreng, on A549 lung cancer cells in relation to apoptotic mechanistic pathway. Girinimbine was isolated from Murraya koenigii Spreng. The antiproliferative activity was assayed using MTT and the apoptosis detection was done by annexin V and lysosomal stability assays. Multiparameter cytotoxicity assays were performed to investigate the change in mitochondrial membrane potential and cytochrome c translocation. ROS, caspase, and human apoptosis proteome profiler assays were done to investigate the apoptotic mechanism of cell death. The MTT assay revealed that the Girinimbine induces cell death with an IC50 of 19.01 mu M. A significant induction of early phase of apoptosis was shown by annexin V and lysosomal stability assays. After 24 h treatment with 19.01 mu M of Girinimbine, decrease in the nuclear area and increase in mitochondrial membrane potential and plasma membrane permeability were readily visible. Moreover the translocation of cytochrome c also was observed. Girinimbine mediates its antiproliferative and apoptotic effects through up- and downregulation of apoptotic and antiapoptotic proteins. There was a significant involvement of both intrinsic and extrinsic pathways. Moreover, the upregulation of p53 as well as the cell proliferation repressor proteins, p27 and p21, and the significant role of insulin/IGF-1 signaling were also identified. Moreover the caspases 3 and 8 were found to be significantly activated. Our results taken together indicated that Girinimbine may be a potential agent for anticancer drug development.
Chemical constituents from stem bark and roots of Clausena anisata.[Pubmed:23169265]
Molecules. 2012 Nov 20;17(11):13673-86.
Phytochemical investigations on the stem bark and roots of the tropical shrub Clausena anisata led to the isolation and characterization three carbazole alkaloids: Girinimbine, murrayamine-A and ekeberginine; two peptide derivatives: aurantiamide acetate and N-benzoyl-L-phenylalaninyl-N-benzoyl-L-phenylalaninate; and a mixture of two phytosterols: sitosterol and stigmasterol. The structures of these compounds were established by nuclear magnetic resonance (1H-NMR, 13C-NMR, COSY, HSQC, HMQC, HMBC and NOESY) spectroscopy and electrospray ionization mass spectrometry (MS).
Anti-tumour promoting activity and antioxidant properties of girinimbine isolated from the stem bark of Murraya koenigii S.[Pubmed:22522395]
Molecules. 2012 Apr 20;17(4):4651-60.
Girinimbine, a carbazole alkaloid isolated from the stem bark of Murraya koenigii was tested for the in vitro anti-tumour promoting and antioxidant activities. Anti-tumour promoting activity was determined by assaying the capability of this compound to inhibit the expression of early antigen of Epstein-Barr virus (EA-EBV) in Raji cells that was induced by the tumour promoter, phorbol 12-myristate 13-acetate. The concentration of this compound that gave an inhibition rate at fifty percent was 6.0 microg/mL and was not cytotoxic to the cells. Immunoblotting analysis of the expression of EA-EBV showed that Girinimbine was able to suppress restricted early antigen (EA-R). However, diffused early antigen (EA-D) was partially suppressed when used at 32.0 microg/mL. Girinimbine exhibited a very strong antioxidant activity as compared to a-tocopherol and was able to inhibit superoxide generation in the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced differentiated premyelocytic HL-60 cells more than 95%, when treated with the compound at 5.3 and 26.3 microg/mL, respectively. However Girinimbine failed to scavenge the stable diphenyl picryl hydrazyl (DPPH)-free radical.
A new carbazole alkaloid from the leaves of Malayan Murraya koenigii.[Pubmed:21972815]
J Asian Nat Prod Res. 2011 Oct;13(10):972-5.
New carbazole alkaloid, 7-hydroxymurrayazolinine (1), was isolated from the ethanol extract of the leaves of Malayan Murraya koenigii, together with five known carbazole alkaloids, mahanimbine (2), bicyclomahanimbine (3), Girinimbine (4), koenimbine (5), and murrayamine-D (6). Their structures were elucidated on the basis of spectroscopic analysis.
The growth suppressing effects of girinimbine on HepG2 involve induction of apoptosis and cell cycle arrest.[Pubmed:21862957]
Molecules. 2011 Aug 23;16(8):7155-70.
Murraya koenigii is an edible herb widely used in folk medicine. Here we report that Girinimbine, a carbazole alkaloid isolated from this plant, inhibited the growth and induced apoptosis in human hepatocellular carcinoma, HepG2 cells. The MTT and LDH assay results showed that Girinimbine decreased cell viability and increased cytotoxicity in a dose-and time-dependent manner selectively. Girinimbine-treated HepG2 cells showed typical morphological features of apoptosis, as observed from normal inverted microscopy and Hoechst 33342 assay. Furthermore, Girinimbine treatment resulted in DNA fragmentation and elevated levels of caspase-3 in HepG2 cells. Girinimbine treatment also displayed a time-dependent accumulation of the Sub-G(0)/G(1) peak (hypodiploid) and caused G(0)/G(1)-phase arrest. Together, these results demonstrated for the first time that Girinimbine could effectively induce programmed cell death in HepG2 cells and suggests the importance of conducting further investigations in preclinical human hepatocellular carcinoma models, especially on in vivo efficacy, to promote Girinimbine for use as an anticancer agent against hepatocellular carcinoma.
Efficient iron-mediated approach to pyrano[3,2-a]carbazole alkaloids--first total syntheses of O-methylmurrayamine A and 7-methoxymurrayacine, first asymmetric synthesis and assignment of the absolute configuration of (-)-trans-dihydroxygirinimbine.[Pubmed:21279228]
Org Biomol Chem. 2011 Apr 7;9(7):2057-61.
Iron-mediated oxidative cyclisation provides an efficient approach to pyrano[3,2-a]carbazole alkaloids. Thus, improved routes to Girinimbine and murrayacine as well as the first total syntheses of O-methylmurrayamine A and 7-methoxymurrayacine are reported. Asymmetric epoxidation of Girinimbine led to (-)-trans-dihydroxyGirinimbine and the assignment of its absolute configuration.
Murrayakoeninol--a new carbazole alkaloid from Murraya koenigii (Linn) Spreng.[Pubmed:19413112]
Nat Prod Commun. 2009 Mar;4(3):355-8.
A new carbazole alkaloid, designated as murrayakoeninol, was isolated from the leaves of Murraya koenigii (Linn) Spreng, along with four known carbazole alkaloids, viz. mahanimbine, koenimbine, O-methylmurrayamine-A and murrayazolinine and one from the bark viz. Girinimbine. The structure of the new alkaloid 1 was elucidated on the basis of 2D NMR spectral analysis and chemical reactions.


