GeraldolCAS# 21511-25-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21511-25-1 | SDF | Download SDF |
| PubChem ID | 5482101 | Appearance | Powder |
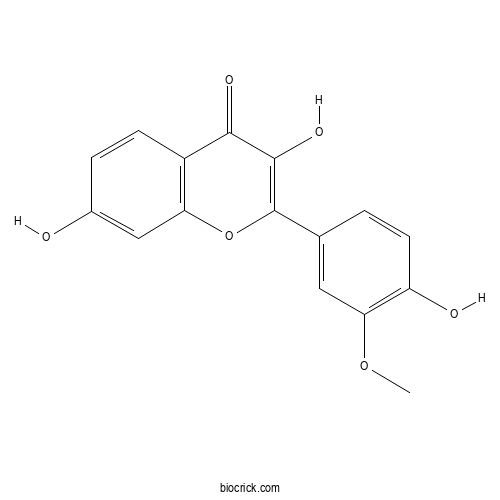
| Formula | C16H12O6 | M.Wt | 300.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=C(C=CC(=C1)C2=C(C(=O)C3=C(O2)C=C(C=C3)O)O)O | ||
| Standard InChIKey | WRFQRUBJBPLPAM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O6/c1-21-13-6-8(2-5-11(13)18)16-15(20)14(19)10-4-3-9(17)7-12(10)22-16/h2-7,17-18,20H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fisetin is an anticancer agent with antiangiogenic properties in mice, it is reported to suppress endothelial cell migration and proliferation. | |||||

Geraldol Dilution Calculator

Geraldol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DL-Malic acid
Catalog No.:BCN9917
CAS No.:617-48-1
- Crotonic acid
Catalog No.:BCN9916
CAS No.:107-93-7
- Tigogenin acetate
Catalog No.:BCN9915
CAS No.:2530-07-6
- (-)-Sparteine
Catalog No.:BCN9914
CAS No.:90-39-1
- Daucoidin A
Catalog No.:BCN9913
CAS No.:103629-87-4
- (R)-O-isobutyroyllomatin
Catalog No.:BCN9912
CAS No.:440094-38-2
- 5-Methoxypiperonal
Catalog No.:BCN9911
CAS No.:5780-07-4
- Corynanthine
Catalog No.:BCN9910
CAS No.:483-10-3
- Epoxybergamottin
Catalog No.:BCN9729
CAS No.:206978-14-5
- Calycopterin
Catalog No.:BCN9907
CAS No.:481-52-7
- Eupalitin 3-galactoside
Catalog No.:BCN9906
CAS No.:35399-32-7
- Eupalitin
Catalog No.:BCN9905
CAS No.:29536-41-2
- Trioxsalen
Catalog No.:BCN9919
CAS No.:3902-71-4
- Quinizarin
Catalog No.:BCN9920
CAS No.:81-64-1
- 3'',4'',5,7-Tetrahydroxy 3,6,8-trimethoxyflavone
Catalog No.:BCN9921
CAS No.:61451-85-2
- (1S)-Chrysanthemolactone
Catalog No.:BCN9922
CAS No.:14087-71-9
- 2-(Beta-D-Glucopyranosyloxy)benzaldehyde
Catalog No.:BCN9923
CAS No.:618-65-5
- Demissidine
Catalog No.:BCN9924
CAS No.:474-08-8
- Furanoeudesma 1,3-diene
Catalog No.:BCN9925
CAS No.:87605-93-4
- trans-Stilbene
Catalog No.:BCN9926
CAS No.:103-30-0
- Sanguinarine nitrate
Catalog No.:BCN9927
CAS No.:4752-86-7
- 4-Phenylmorpholine
Catalog No.:BCN9928
CAS No.:92-53-5
- (+)-Dihydrocinchonine
Catalog No.:BCN9929
CAS No.:485-65-4
- 6-Methoxyflavone
Catalog No.:BCN9930
CAS No.:26964-24-9
Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates.[Pubmed:32380641]
Int J Mol Sci. 2020 May 5;21(9). pii: ijms21093256.
Flavonoids are natural phenolic compounds, which are the active ingredients in several dietary supplements. It is well-known that some flavonoid aglycones are potent inhibitors of the xanthine oxidase (XO)-catalyzed uric acid formation in vitro. However, the effects of conjugated flavonoid metabolites are poorly characterized. Furthermore, the inhibition of XO-catalyzed 6-mercaptopurine oxidation is an important reaction in the pharmacokinetics of this antitumor drug. The inhibitory effects of some compounds on xanthine vs. 6-mercaptopurine oxidation showed large differences. Nevertheless, we have only limited information regarding the impact of flavonoids on 6-mercaptopurine oxidation. In this study, we examined the interactions of flavonoid aglycones and some of their conjugates with XO-catalyzed xanthine and 6-mercaptopurine oxidation in vitro. Diosmetin was the strongest inhibitor of uric acid formation, while apigenin showed the highest effect on 6-thiouric acid production. Kaempferol, fisetin, Geraldol, luteolin, diosmetin, and chrysoeriol proved to be similarly strong inhibitors of xanthine and 6-mercaptopurine oxidation. While apigenin, chrysin, and chrysin-7-sulfate were more potent inhibitors of 6-mercaptopurine than xanthine oxidation. Many flavonoids showed similar or stronger (even 5- to 40-fold) inhibition of XO than the positive control allopurinol. Based on these observations, the extremely high intake of flavonoids may interfere with the elimination of 6-mercaptopurine.
Investigation of chemical profile, biological properties of Lotus corniculatus L. extracts and their apoptotic-autophagic effects on breast cancer cells.[Pubmed:31185340]
J Pharm Biomed Anal. 2019 Sep 10;174:286-299.
This study aimed to reveal chemical profiles and biological activities of ethyl acetate (EA), methanol (MeOH), and water extracts of Lotus corniculatus. Ethnobotanical reports have indicated the importance of phytochemical properties of the genus Lotus. In this study, the effects of medicinal plant extracts on antioxidant (DPPH, ABTS, CUPRAC, FRAP, phosphomolybdenum, and metal chelating assays), enzyme inhibitory (on cholinesterase, tyrosinase, a-amylase and a-glucosidase), DNA protection and anticancer properties (including anti-proliferative, cell death and telomerase activity marker gene analysis, apoptotic DNA fragmentation analysis, cell migration test) were evaluated. According to chemical analysis, quercetin derivatives Geraldol, isorhamnetin and kaempferol-O-coumaroylhexoside-O-deoxyhexoside isomers were dominant in the extracts. MeOH extracts showed the highest total flavonoids capacity with 21.13mg RE/g. EA extract showed the strongest anti-amylase activity among the tested extracts. Water extract had the most protective activity against plasmid DNA. To indicate cell survival, MTT test was performed against human MCF-7 and MDA-MB-231 breast cancer cells. Half-maximal inhibitory concentration for cells were calculated and used for detection of mechanisms behind the cancer cell death. EA extract showed up-regulation of Bax proapoptotic gene and apoptotic DNA fragmentation activity on highly invasive MDA-MB-231 cells. Beclin-1 and LC3-II autophagy genes were higly expressed after treatment of MCF-7 cells with EA extracts. EA and MeOH extracts inhibited cell migration ability of both cancer cells. Linoleamide, was dominant component in EA extract and caused apoptosis on MDA-MB-231 breast cancer cells via increasing intranuclear Ca(2)(+). The detailed mechanism behind the anticancer properties should be further investigated.
Selective inhibition of CYP2C8 by fisetin and its methylated metabolite, geraldol, in human liver microsomes.[Pubmed:29454704]
Drug Metab Pharmacokinet. 2018 Apr;33(2):111-117.
Fisetin is a flavonol compound commonly found in edible vegetables and fruits. It has anti-tumor, antioxidant, and anti-inflammatory effects. Geraldol, the O-methyl metabolite of fisetin in mice, is reported to suppress endothelial cell migration and proliferation. Although the in vivo and in vitro effects of fisetin and its metabolites are frequently reported, studies on herb-drug interactions have not yet been performed. This study was designed to investigate the inhibitory effect of fisetin and Geraldol on eight isoforms of human cytochrome P450 (CYP) by using cocktail assay and LC-MS/MS analysis. The selective inhibition of CYP2C8-catalyzed paclitaxel hydroxylation by fisetin and Geraldol were confirmed in pooled human liver microsomes (HLMs). In addition, an IC50 shift assay under different pre-incubation conditions confirmed that fisetin and Geraldol shows a reversible concentration-dependent, but not mechanism-based, inhibition of CYP2C8. Moreover, Michaelis-Menten, Lineweaver-burk plots, Dixon and Eadie-Hofstee showed a non-competitive inhibition mode with an equilibrium dissociation constant of 4.1 muM for fisetin and 11.5 muM for Geraldol, determined from secondary plot of the Lineweaver-Burk plot. In conclusion, our results indicate that fisetin showed selective reversible and non-competitive inhibition of CYP2C8 more than its main metabolite, Geraldol, in HLMs.
Identification of absolute conversion to geraldol from fisetin and pharmacokinetics in mouse.[Pubmed:27810278]
J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Dec 1;1038:95-100.
Fisetin (3,3',4',7-tetrahydroxyflavone) is a flavonoid found in several fruits, vegetables, nuts, and wine and has anti-oxidant, anti-inflammatory, and anti-angiogenic properties. Geraldol is the 3'-methoxylated metabolite of fisetin (3,4',7-trihydroxy-3'-methoxyflavone). The concentration of fisetin and Geraldol in mouse plasma was determined by LC-MS/MS, following direct protein precipitation. These concentrations were determined after administration of fisetin at doses of 2mg/kg (i.v.) and 100 and 200mg/kg (p.o.). The method was validated in terms of linearity, accuracy, precision, matrix effect, and stability. The pharmacokinetics parameters of fisetin and Geraldol were successfully determined using a validated method in mice. Results indicated that fisetin was very rapidly methylated to Geraldol in vivo. Following administration of fisetin, it was observed that the Cmax and AUC values for Geraldol were higher than those of fisetin. The absolute bioavailability of fisetin was calculated as 7.8% and 31.7% after oral administration of 100 and 200mg/kg fisetin, respectively. This method was successfully applied to determine the pharmacokinetic parameters of fisetin and its main metabolite Geraldol in mouse plasma. Geraldol was the dominant circulating metabolite after fisetin administration in vivo.
Structure related effects of flavonoid aglycones on cell cycle progression of HepG2 cells: Metabolic activation of fisetin and quercetin by catechol-O-methyltransferase (COMT).[Pubmed:27522262]
Biomed Pharmacother. 2016 Oct;83:998-1005.
Dietary flavonoids are abundant in the Plant Kingdom and they are extensively studied because of their manifold pharmacological activities. Recent studies highlighted that cell cycle arrest plays a key role in their antiproliferative effect in different tumor cells. However, structure-activity relationship of flavonoids is poorly characterized. In our study the influence of 18 flavonoid aglycones (as well as two metabolites) on cell cycle distribution was investigated. Since flavonoids are extensively metabolized by liver cells, HepG2 tumor cell line was applied, considering the potential metabolic activation/inactivation of flavonoids. Our major observations are the followings: (1) Among the tested compounds diosmetin, fisetin, apigenin, lutelin, and quercetin provoked spectacular extent of G2/M phase cell cycle arrest. (2) Inhibition of catechol-O-methyltransferase enzyme by entacapone decreased the antiproliferative effects of fisetin and quercetin. (3) Geraldol and isorhamnetin (3'-O-methylated metabolites of fisetin and quercetin, respectively) demonstrated significantly higher antiproliferative effect on HepG2 cells compared to the parent compounds. Based on these results, O-methylated flavonoid metabolites or their chemically modified derivatives may be suitable candidates of tumor therapy in the future.
Radical scavenging activities of flavonoids from roots of Akschindlium godefroyanum.[Pubmed:25426867]
Nat Prod Res. 2015;29(12):1177-9.
Chemical constituents of crude ethyl acetate extract of roots of Akschindlium godefroyanum (Kuntze) H. Ohashi were investigated and seven flavonoids were isolated. Their structures were identified based on spectroscopic methods as well as by comparison with spectral data reported in the literature as six flavanonols and a flavonol including 7,4'-dihydroxy-5,3'-dimethoxyflavanonol (1), neophellamuretin (2), taxifolin (3), erycibenin D (4), Geraldol (5), fustin (6) and garbanzol (7). Compounds 2, 4 and 7 were found in the genus Akschindlium for the first time. Compounds 3, 5 and 6 appeared to have free radical scavenging activities using DPPH assay with IC50 of 21, 40 and 15 mug/mL, respectively.
Two new flavanonols from the bark of Akschindlium godefroyanum.[Pubmed:24354343]
Nat Prod Res. 2014;28(3):191-5.
The first phytochemical investigation of the bark of Akschindlium godefroyanum (Kuntze) H. Ohashi (Fabaceae), a Thai herbal medicine, resulted in the isolation of two new flavanonols, 7,3',5'-trihydroxy-5-methoxyflavanonol (1) and 7,4'-dihydroxy-5,3'-dimethoxyflavanonol (2), and eight known compounds comprising one flavonol, Geraldol (3); three flavanonols, (+)-taxifolin (4), (+)-fustin (5) and aromadendrin 5-methyl ether (6); one catechin, (-)-epigallocatechin (7); one triterpenoid, lupeol (8); one steroid, stigmasterol (9) and one steroid glycoside, stigmasterol-3-O-beta-d-glucoside (10). Their structures were identified by spectroscopic methods.
Small molecule inhibitors of the HPV16-E6 interaction with caspase 8.[Pubmed:22300659]
Bioorg Med Chem Lett. 2012 Mar 1;22(5):2125-9.
High-risk strains of human papillomaviruses (HPVs) cause nearly all cases of cervical cancer as well as a growing number of head and neck cancers. The oncogenicity of these viruses can be attributed to the activities of their two primary oncoproteins, E6 and E7. The E6 protein has among its functions the ability to prevent apoptosis of infected cells through its binding to FADD and caspase 8. A small molecule library was screened for candidates that could inhibit E6 binding to FADD and caspase 8. Flavonols were found to possess this activity with the rank order of myricetin>morin>quercetin>kaempferol=galangin>>(apigenin, 7-hydroxyflavonol, rhamnetin, isorhamnetin, Geraldol, datiscetin, fisetin, 6-hydroxyflavonol). Counter screening, where the ability of these chosen flavonols to inhibit caspase 8 binding to itself was assessed, demonstrated that myricetin, morin and quercetin inhibited GST-E6 and His-caspase 8 binding in a specific manner. The structure-activity relationships suggested by these data are unique and do not match prior reports on flavonols in the literature for a variety of anticancer assays.
Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite.[Pubmed:21840301]
Biochem Pharmacol. 2011 Dec 1;82(11):1731-9.
Although the natural flavonoid fisetin (3,3',4',7-tetrahydroxyflavone) has been recently identified as an anticancer agent with antiangiogenic properties in mice, its in vivo pharmacokinetics and metabolism are presently not characterized. Our purpose was to determine the pharmacokinetics and metabolism of fisetin in mice and determine the biological activity of a detected fisetin metabolite. After fisetin administration of an efficacious dose of 223 mg/kg i.p. in mice, the maximum fisetin concentration reached 2.5 mug/ml at 15 min and the plasma concentration declined biphasically with a rapid half-life of 0.09 h and a terminal half-life of 3.1h. Three metabolites were detected, one of which was a glucuronide of fisetin (M1), whereas another glucuronide (M2) was a glucuronide of a previously unknown fisetin metabolite (M3). HPLC-MS/MS analysis indicated that M3 was a methoxylated metabolite of fisetin (MW=300 Da). The UV spectrum of M3 was identical to that of fisetin and standard 3,4',7-trihydroxy-3'-methoxyflavone (Geraldol). In addition, because M3 co-eluted with standard Geraldol in 4 different chromatographic ternary gradient conditions, M3 was therefore assigned to Geraldol. Of interest, this metabolite was shown to achieve higher concentrations than fisetin in Lewis lung tumors. We also compared the cytotoxic and antiangiogenic activities of fisetin and Geraldol in vitro and it was found that the latter was more cytotoxic than the parent compound toward tumor cells, and that it could also inhibit endothelial cells migration and proliferation. In conclusion, these results suggest that fisetin metabolism plays an important role in its in vivo anticancer activities.
Caspase-3-dependent mitotic checkpoint inactivation by the small-molecule inducers of mitotic slippage SU6656 and geraldol.[Pubmed:21441410]
Mol Cancer Ther. 2011 May;10(5):839-49.
Microtubule-targeting cancer drugs such as paclitaxel block cell-cycle progression at mitosis by prolonged activation of the mitotic checkpoint. Cells can spontaneously escape mitotic arrest and enter interphase without chromosome segregation by a process termed mitotic slippage that involves the degradation of cyclin B1 without mitotic checkpoint inactivation. Inducing mitotic slippage with chemicals causes cells to die after multiple rounds of DNA replication without cell division, which may enhance the antitumor activity of microtubule-targeting drugs. Here, we explore pathways leading to mitotic slippage by using SU6656 and Geraldol, two recently identified chemical inducers of mitotic slippage. Mitotic slippage induced by SU6656 or Geraldol was blocked by the proteasome inhibitor MG-132 and involved proteasome-dependent degradation of cyclin B1 and the mitotic checkpoint proteins budding uninhibited by benzimidazole related 1 (BubR1) and cell division cycle 20 (Cdc20) in T98G cells. Mitotic slippage and the degradation of BubR1 and Cdc20 were also inhibited by the caspase-3 and -7 inhibitor DEVD-CHO. MCF-7 cells lacking caspase-3 expression could not degrade BubR1 or undergo mitotic slippage in response to SU6656 or Geraldol. Introduction of caspase-3 completely restored the ability of MCF-7 cells to degrade BubR1 and undergo mitotic slippage. However, lack of expression of caspase-3 did not affect cell death after exposure to paclitaxel, with or without mitotic slippage induction. The requirement for caspase-3 for chemically induced mitotic slippage reveals a new mechanism for mitotic exit and a link between mitosis and apoptosis that has implications for the outcome of cancer chemotherapy.
Effects of chemical manipulation of mitotic arrest and slippage on cancer cell survival and proliferation.[Pubmed:19713760]
Cell Cycle. 2009 Sep 15;8(18):3025-38. Epub 2009 Sep 25.
Microtubule-targeting cancer therapies interfere with mitotic spindle dynamics and block cells in mitosis by activating the mitotic checkpoint. Cells arrested in mitosis may remain arrested for extended periods of time or undergo mitotic slippage and enter interphase without having separated their chromosomes. How extended mitotic arrest and mitotic slippage contribute to subsequent cell death or survival is incompletely understood. To address this question, automated fluorescence microscopy assays were designed and used to screen chemical libraries for modulators of mitotic slippage. Chlorpromazine and triflupromazine were identified as drugs that inhibit mitotic slippage and SU6656 and Geraldol as chemicals that stimulate mitotic slippage. Using the drugs to extend mitotic arrest imposed by low concentrations of paclitaxel led to increased cell survival and proliferation after drug removal. Cells arrested at mitosis with paclitaxel or vinblastine and chemically induced to undergo mitotic slippage underwent several rounds of DNA replication without cell division and exhibited signs of senescence but eventually all died. By contrast, cells arrested at mitosis with the KSP/Eg5 inhibitor S-trityl-L-cysteine and induced to undergo mitotic slippage were able to successfully divide and continued to proliferate after drug removal. These results show that reinforcing mitotic arrest with drugs that inhibit mitotic slippage can lead to increased cell survival and proliferation, while inducing mitotic slippage in cells treated with microtubule-targeting drugs seems to lead to protracted cell death.
[Effects of flavonoids with different structures on proliferation of leukemia cell line HL-60].[Pubmed:18076792]
Ai Zheng. 2007 Dec;26(12):1309-14.
BACKGROUND & OBJECTIVE: Flavonoids, with some beneficial biological activities, exist extensively in foods and herbal products. This study was to evaluate the effects of 23 flavonoids on the proliferation of leukemia cell line HL-60, and elucidate the structure-activity relationship (SAR). METHODS: HL-60 cells were treated with 23 flavonoids with high purity and definite structure. Cell proliferation was detected by MTT assay. The 50% inhibition concentrations (IC50) of the 23 flavonoids were calculated. The effects of particular structures on IC50 were evaluated. RESULTS: Most of the 23 flavonoids inhibited the proliferation of HL-60 cells distinctly, and the effects were enhanced along with increasing concentrations. However, the intensity of their effects were different, which were arranged from strong to weak as follows:3,6-dihydroxyflavone > luteolin > Geraldol > 2'-hydroxyflavanone > apigenin > 3,7-dihydroxyflavone > myricetin > fisetin > baicalein > quercetin > flavanone > chrysin > galangin > 4'-hydroxyflavanone > 6-hydroxyflavone > genistein > flavone >7-hydroxyflavone > daidzein > hesperetin > naringenin. The 2,3-double bond in ring C, appropriate hydroxyls, ring B attached at position 2, hydroxyls in position 3, ortho-substituting hydroxyls in ring B were related to enhanced inhibitory effects of flavonoids on the proliferation of HL-60 cells, while the lack of 2,3-double bond, deficiency or redundancy of hydroxyl groups, hydroxyl group in position 5, 7 or meta-substituting hydroxyls in ring B, isoflavone structure were related to reduced inhibitory effects of flavonoids. CONCLUSION: The 2,3-double bond in ring C, appropriate hydroxyls, ring B attached at position 2, hydroxyls in position 3, ortho-substituting hydroxyls in ring B may be key structural requirements of flavonoids for potent cytotoxicity to HL-60 cells.


