Genistein 7-O-glucuronideCAS# 38482-81-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38482-81-4 | SDF | Download SDF |
| PubChem ID | 15940724 | Appearance | Powder |
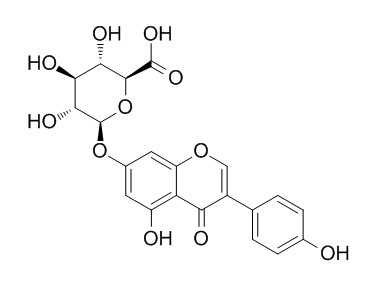
| Formula | C21H18O11 | M.Wt | 446.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-[5-hydroxy-3-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid | ||
| SMILES | C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)OC4C(C(C(C(O4)C(=O)O)O)O)O)O | ||
| Standard InChIKey | JIVINIISUDEORF-ZFORQUDYSA-N | ||
| Standard InChI | InChI=1S/C21H18O11/c22-9-3-1-8(2-4-9)11-7-30-13-6-10(5-12(23)14(13)15(11)24)31-21-18(27)16(25)17(26)19(32-21)20(28)29/h1-7,16-19,21-23,25-27H,(H,28,29)/t16-,17-,18+,19-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Genistein 7-O-glucuronide has estrogenic potency. | |||||

Genistein 7-O-glucuronide Dilution Calculator

Genistein 7-O-glucuronide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2401 mL | 11.2007 mL | 22.4014 mL | 44.8029 mL | 56.0036 mL |
| 5 mM | 0.448 mL | 2.2401 mL | 4.4803 mL | 8.9606 mL | 11.2007 mL |
| 10 mM | 0.224 mL | 1.1201 mL | 2.2401 mL | 4.4803 mL | 5.6004 mL |
| 50 mM | 0.0448 mL | 0.224 mL | 0.448 mL | 0.8961 mL | 1.1201 mL |
| 100 mM | 0.0224 mL | 0.112 mL | 0.224 mL | 0.448 mL | 0.56 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxyacanthine sulfate
Catalog No.:BCN9966
CAS No.:6183-91-1
- Cubebin
Catalog No.:BCN9965
CAS No.:1242843-00-0
- 2,3-Dehydrosilybin B
Catalog No.:BCN9964
CAS No.:142796-24-5
- 4'-Methylchrysoeriol
Catalog No.:BCN9963
CAS No.:4712-12-3
- Aegineoside
Catalog No.:BCN9962
CAS No.:752209-48-6
- Oleocanthal
Catalog No.:BCN9961
CAS No.:289030-99-5
- 2',6'-Dihydroxy 4'-methoxydihydrochalcone
Catalog No.:BCN9960
CAS No.:35241-55-5
- Withanone
Catalog No.:BCN9959
CAS No.:27570-38-3
- Citronellyl acetate
Catalog No.:BCN9958
CAS No.:150-84-5
- p-Mentha-1,4-diene
Catalog No.:BCN9957
CAS No.:99-85-4
- Isogentisin
Catalog No.:BCN9956
CAS No.:491-64-5
- 4-Methylcatechol
Catalog No.:BCN9955
CAS No.:452-86-8
- 4-Ethoxycoumarin
Catalog No.:BCN9968
CAS No.:35817-27-7
- Psoromic acid
Catalog No.:BCN9969
CAS No.:7299-11-8
- Butyric acid
Catalog No.:BCN9970
CAS No.:107-92-6
- Vescalagin
Catalog No.:BCN9971
CAS No.:36001-47-5
- Terpinyl acetate
Catalog No.:BCN9972
CAS No.:80-26-2
- Peltatoside
Catalog No.:BCN9973
CAS No.:23284-18-6
- Platycogenin A
Catalog No.:BCN9974
CAS No.:1459719-53-9
- 9-Methyl-9-azabicyclo[3.3.1]nonan-3-one
Catalog No.:BCN9975
CAS No.:552-70-5
- AT101
Catalog No.:BCN9976
CAS No.:866541-93-7
- omega-Benzoyl oxyphloracetophenone
Catalog No.:BCN9977
CAS No.:65982-77-6
- Henricine
Catalog No.:BCN9978
CAS No.:107783-46-0
- Harmol
Catalog No.:BCN9979
CAS No.:487-03-6
Glucuronides of phytoestrogen flavonoid enhance macrophage function via conversion to aglycones by beta-glucuronidase in macrophages.[Pubmed:28480538]
Immun Inflamm Dis. 2017 Sep;5(3):265-279.
INTRODUCTION: Flavonoids are converted to inactive metabolites like glucuronides in the gut, and circulate mainly as glucuronides in blood stream, resulting in low concentrations of active aglycones in plasma. It is therefore unclear how oral flavonoids exert their effects in tissues. We recently reported the plasma pharmacokinetics of some flavonoids and suggested the possibility that the absorbed flavonoids modified macrophage functions leading to enhance bacterial clearance. We aimed to confirm their pharmacological profiles focusing on tissue macrophages. METHODS: Pseudoinfection was induced by intradermal injection of FITC-conjugated and killed Staphylococcus aureus into the ears of mice treated with or without Genistein 7-O-glucuronide (GEN7G, 1 mg/kg, i.v.). FACS analysis was performed on single cell suspensions dispersed enzymatically from the skin lesions at 6 h post pseudoinfection to evaluate phagocytic activities of monocytes/macrophages (CD11b(+) Ly6G(-) ) and neutrophils (CD11b(+) Ly6G(+) ). Phagocytosis of the FITC-conjugated bacteria by four glucuronides including GEN7G was evaluated in cultures of mouse macrophages. RESULTS: After GEN7G injection, genistein was identified in the inflamed ears as well as GEN7G, and the phagocytic activity of CD11b(+) Ly6G(-) cells was increased. GEN7G was converted to genistein by incubation with macrophage-related beta-glucuronidase. Macrophage culture assays revealed that GEN7G increased phagocytosis, and the action was dampened by a beta-glucuronidase inhibitor. Binding of aglycones to estrogen receptors (ERs), putative receptors of flavonoid aglycones, correlated to biological activities, and glucuronidation reduced the binding to ERs. An ER antagonist suppressed the increase of macrophage function by GEN7G, whereas estradiol enhanced phagocytosis as well. CONCLUSIONS: This study suggests a molecular mechanism by which oral flavonoids are carried as glucuronides and activated to aglycones by beta-glucuronidase in tissue macrophages, and contributes to the pharmacological study of glucuronides.
Plasma Pharmacokinetics of Polyphenols in a Traditional Japanese Medicine, Jumihaidokuto, Which Suppresses Propionibacterium acnes-Induced Dermatitis in Rats.[Pubmed:26437394]
Molecules. 2015 Sep 30;20(10):18031-46.
Most orally administered polyphenols are metabolized, with very little absorbed as aglycones and/or unchanged forms. Metabolic and pharmacokinetic studies are therefore necessary to understand the pharmacological mechanisms of polyphenols. Jumihaidokuto (JHT), a traditional Japanese medicine, has been used for treatment of skin diseases including inflammatory acne. Because JHT contains various types of bioactive polyphenols, our aim was to clarify the metabolism and pharmacokinetics of the polyphenols in JHT and identify active metabolites contributing to its antidermatitis effects. Orally administered JHT inhibited the increase in ear thickness in rats induced by intradermal injection of Propionibacterium acnes. Quantification by LC-MS/MS indicated that JHT contains various types of flavonoids and is also rich in hydrolysable tannins, such as 1,2,3,4,6-penta-O-galloyl glucose. Pharmacokinetic and antioxidant analyses showed that some flavonoid conjugates, such as Genistein 7-O-glucuronide and liquiritigenin 7-O-glucuronide, appeared in rat plasma and had an activity to inhibit hydrogen peroxide-dependent oxidation. Furthermore, 4-O-methylgallic acid, a metabolite of Gallic acid, appeared in rat plasma and inhibited the nitric oxide reaction. JHT has numerous polyphenols; it inhibited dermatitis probably via the antioxidant effect of its metabolites. Our study is beneficial for understanding in vivo actions of orally administered polyphenol drugs.
The kinetic basis for age-associated changes in quercetin and genistein glucuronidation by rat liver microsomes.[Pubmed:19446449]
J Nutr Biochem. 2010 Jun;21(6):498-503.
The dietary bioavailability of the isoflavone genistein is decreased in older rats compared to young adults. Since flavonoids are metabolized extensively by the UDP-glucuronosyltransferases (UGTs), we hypothesized that UGT flavonoid conjugating activity changes with age. The effect of age on flavonoid glucuronidation was determined using hepatic microsomes from male F344 rats. Kinetic models of UGT activity toward the flavonol quercetin and the isoflavone genistein were established using pooled hepatic microsomal fractions of rats at different ages, and glucuronidation rates were determined using individual samples. Intrinsic clearance (V(max)/K(m)) values in 4-, 18- and 28-month-old rats were 0.100, 0.078 and 0.087 ml/min/mg for quercetin-7-O-glucuronide; 0.138, 0.133 and 0.088 for quercetin-3'-O-glucuronide; and 0.075, 0.077 and 0.057 for quercetin-4'-O-glucuronide, respectively. While there were no differences in formation rates of total quercetin glucuronides in individual samples, the production of the primary metabolite, quercetin-7-O-glucuronide, at 30 microM quercetin concentration was increased from 3.4 and 3.1 nmol/min/mg at 4 and 18 months to 3.8 nmol/min/mg at 28 months, while quercetin-3'-O-glucuronide formation at 28 months declined by a similar degree (P
Comparison of genistein metabolism in rats and humans using liver microsomes and hepatocytes.[Pubmed:18063284]
Food Chem Toxicol. 2008 Mar;46(3):939-48.
Species differences and metabolism are the most crucial factors in considering the effects of genistein. The aim of this study was to have a better knowledge of the metabolic fate of genistein in humans as compared with rats. For this purpose, radiolabeled genistein was incubated with human and rat liver microsomes and with cryopreserved hepatocytes from both species. Incubations were performed using a wide range of genistein concentrations to analyze the kinetics of formation of the metabolites. Metabolite profiling was obtained using an HPLC system connected to a radioactivity detector. Identification of the metabolites was based on their retention times as compared with those of authentic standards and on LC-MS (ESI-MS/MS) or NMR analyses. In both species, liver microsomes produced the same three hydroxylated metabolites (8-OH, 6-OH and 3'-OH-genistein) whereas cryopreserved hepatocytes produced the same glucurono- and sulfo-conjugates (genistein 4'-O-sulfate 7-O-glucuronide, Genistein 7-O-glucuronide, genistein 4'-O-glucuronide, genistein 7-O-sulfate and genistein 4'-O-sulfate). The rate of metabolism varied with species. 3'-Hydroxygenistein was the predominant metabolite produced by rat liver microsomes, whereas in humans 3'-hydroxy and 8-hydroxygenistein were produced in the same range. In both human and rat hepatocyte incubations, Genistein 7-O-glucuronide represented more than 50% of the incubated dose. Our results on hepatocytes confirmed the predominance of conjugation reaction compared to oxidative reaction observed in vivo.


